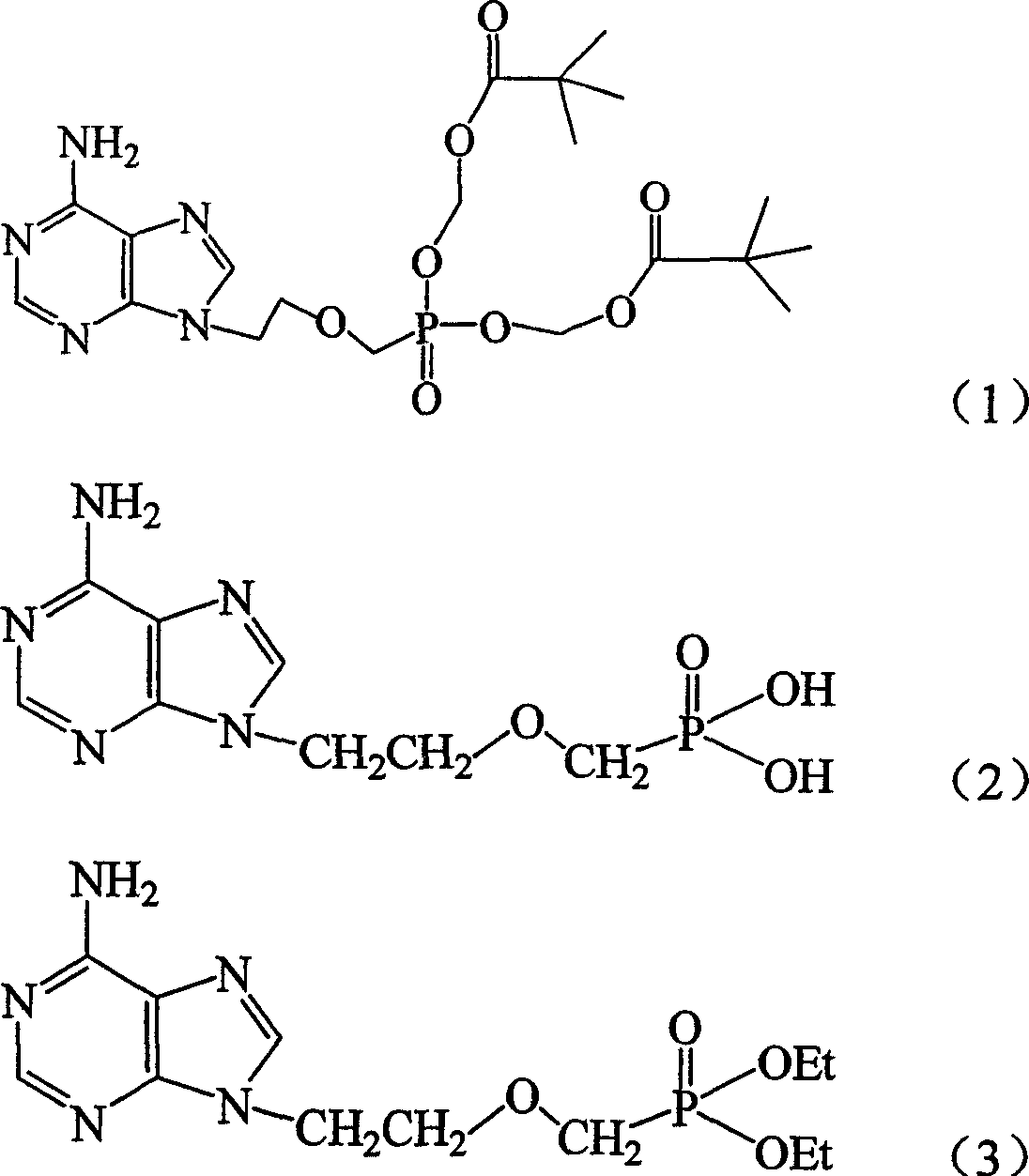
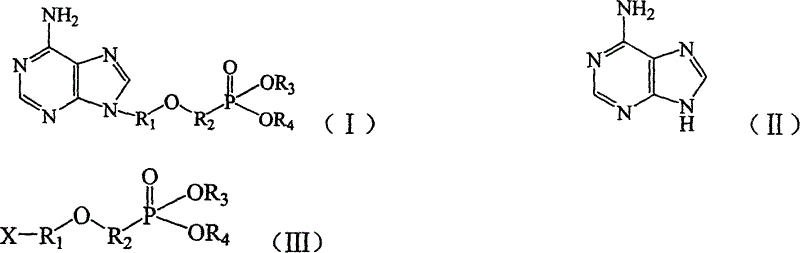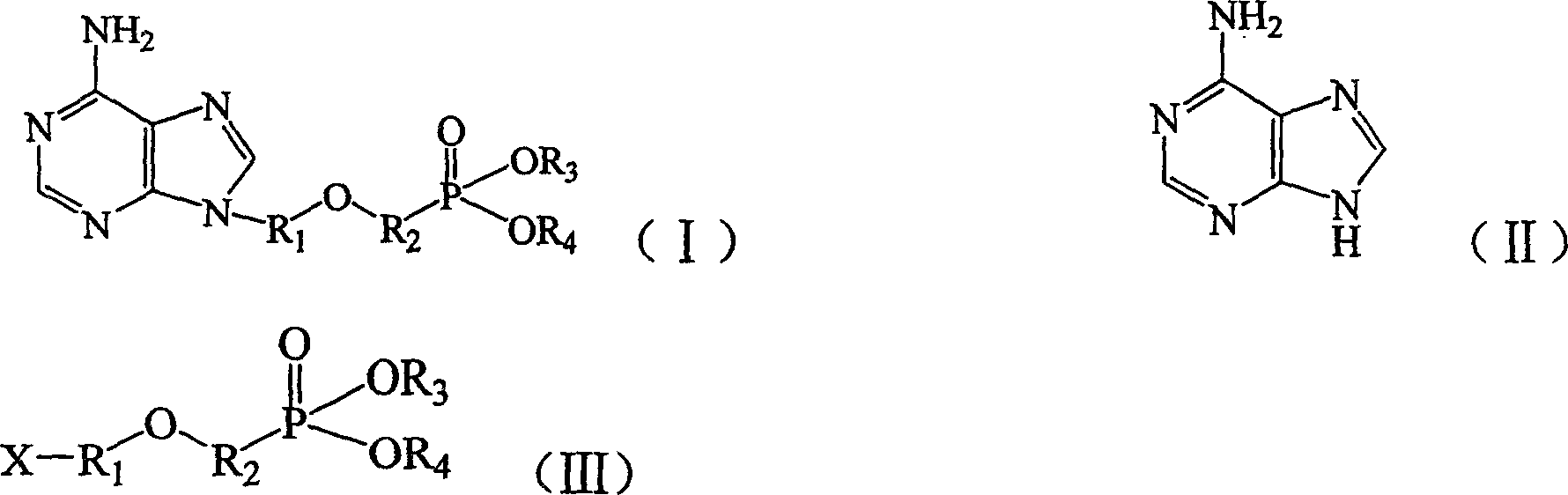Preparation method of adenine derivative
A technology of adenine and derivatives, applied in the field of preparation of adenine derivatives, can solve the problems of low yield and low purity, and achieve the effect of high product purity and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation of 9-(2-diethoxyphosphonomethoxyethyl)adenine
[0021] Throw 54g of adenine, 115g of potassium carbonate, 1g of sodium methoxide, and 600ml of DMF into a 1000ml reaction flask, raise the temperature to 100°C, add 103g of substituted phosphonate such as formula (IV) dropwise, and react at 120°C for 18 Hour. During the reaction, TLC (chloroform:methanol=7:1) was used to track and determine the end point. After the reaction was finished, potassium carbonate was removed by filtration, and the filter cake was washed with an appropriate amount of DMF, and the filter cake was discarded. DMF was recovered under reduced pressure until the material became a paste, 300 g of acetone was added and stirred for 24 hours, the condensate was obtained by filtration, and dried to obtain 118 g of the product with a yield of 89.6%. The obtained product was quantitatively analyzed by high performance liquid chromatography, and the content of 9-(2-diethoxyphosphonometh...
Embodiment 2
[0032]Throw 54g of adenine, 115g of potassium carbonate, 1g of sodium ethoxide, and 600ml of DMF into a 1000ml reaction flask, raise the temperature to 100°C, add 150g of substituted phosphonate such as formula (IV) dropwise, and react at 120°C for 18 Hour. The process was followed by TLC (chloroform:methanol=7:1) and the end point was determined. After the reaction was finished, potassium carbonate was removed by filtration, and the filter cake was washed with an appropriate amount of DMF, and the filter cake was discarded. DMF was recovered under reduced pressure until the material became a paste, 300 g of acetone was added and stirred for 24 hours, the condensate was obtained by filtration, and dried to obtain 100 g of the product with a yield of 76.0%. The chromatographic conditions described in Example 1 were used for quantitative analysis of the obtained product, and the content of 9-(2-diethoxyphosphonomethoxyethyl)adenine was 94%.
Embodiment 3
[0033] Example 3 Preparation of 9-(2-diethoxyphosphonomethoxyethyl)adenine
[0034] Throw 54g of adenine, 150g of potassium carbonate, 2g of sodium methoxide, and 800ml of DMSO into a 1500ml reaction bottle, heat up to 100°C, add 120g of substituted phosphonate as in formula (IV) dropwise, and react at 120°C for 24 Hour. During the reaction, TLC (chloroform:methanol=7:1) was used to track and determine the end point. After the reaction was finished, potassium carbonate was removed by filtration and the filter cake was washed with an appropriate amount of DMSO, and the filter cake was discarded. DMSO was recovered under reduced pressure until the material became a paste, 300 g of acetone was added and stirred for 24 hours, the condensate was obtained by filtration, and dried to obtain 120 g of the product with a yield of 91.1%. The chromatographic conditions described in Example 1 were used for quantitative analysis of the obtained product, and the content of 9-(2-diethoxypho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com