Method for preparing NiB non-crystalline alloy catalyst with the aid of microwave
A technology of amorphous alloys and catalysts, applied in catalyst carriers, chemical instruments and methods, catalyst activation/preparation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6
[0061] These examples illustrate the preparation of unsupported NiB amorphous alloy catalysts.
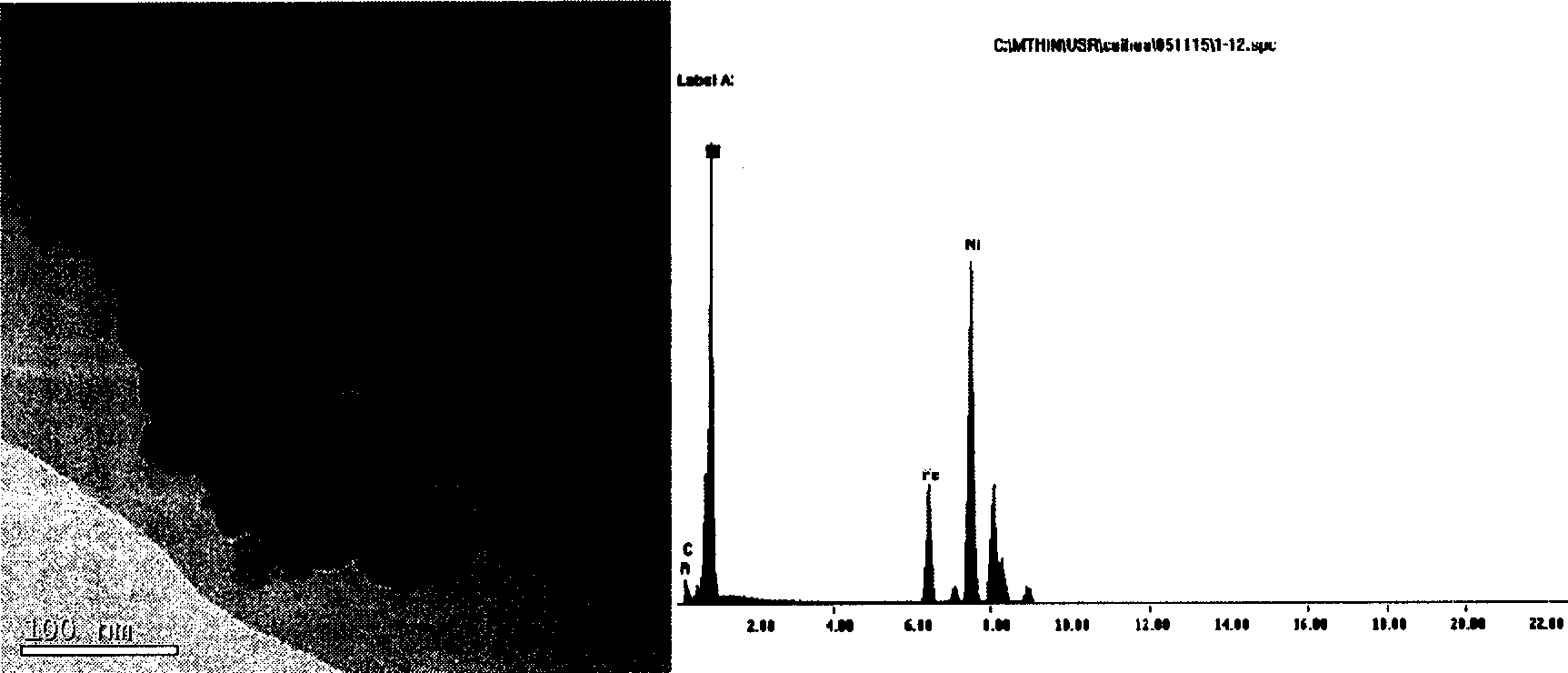
[0062] Configure 0.1mol / L nickel sulfate solution and 1.0mol / L KBH 4 solution (0.01mol / L NaOH to adjust pH=10, B / Ni=2:1, atomic ratio). At 0°C, air or N 2 atmosphere, under ultrasonic vibration, the KBH 4 The solution was added dropwise to the nickel sulfate solution, reacted until no gas was generated, washed with deionized water until neutral, then washed with absolute ethanol, and stored in absolute ethanol, coded as A-C. The samples were washed with water until neutral, then washed with absolute ethanol, and stored in ethanol.
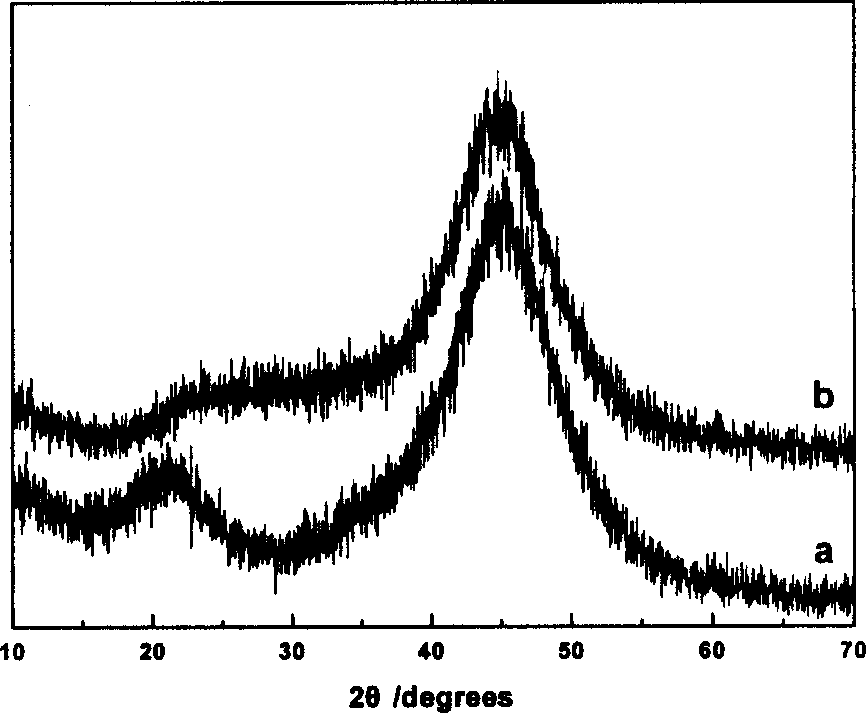
[0063] During the reaction, catalyst A is obtained by using water as a solvent; catalyst B is obtained by using ethylene glycol as a solvent; and catalyst C is obtained by using N,N-dimethylamide as a solvent. The composition of the catalyst is shown in Table 1. The XRD of catalysts A-C have figure 1 a feature.
[0064] The non-supported NiB amor...
Embodiment 7~12
[0087] These examples illustrate the process of ultrasonically assisted preparation of NiB amorphous alloy catalysts using different main salts and co-salts.
[0088] Same as the preparation process of catalyst A, change the main salt nickel sulfate to nickel nitrate, nickel chloride and nickel acetate and keep the metal salt concentration constant to obtain catalyst G-I.
[0089] Using nickel sulfate as the main salt, using iron sulfate as the auxiliary salt, changing the atomic ratio of Ni and Fe (1:1, 2:1, 4:1), and keeping the total concentration of metal ions at 0.1mol / L, prepared according to catalyst A Methods to obtain catalyst K-L.
[0090] The composition of G-L is shown in Table 1.
Embodiment 13~14
[0092] These examples illustrate the preparation of supported NiB amorphous alloy catalysts.
[0093] Chemical reduction method: the carrier used is titanium oxide (Nanjing Titanium Dioxide Co., Ltd.), and the BET specific surface area is 11.5m 2 / g, average pore volume 0.47cm 3 / g, the average pore diameter is 16.2nm. Weigh 200gTiO 2 Powder, baked at 450°C for 4 hours, weighed 5g carrier in 11ml 1.3mol / LNiSO 4 The solution was soaked for 4 hours. The obtained carrier is suction filtered or centrifuged, dried in the air, oven-dried and roasted. At 0°C, under ultrasonic conditions, nitrogen protection, and vigorous stirring, the KBH 4 solution (2mol / L) with the above TiO 2 React until no gas is produced, filter, wash until neutral, and protect with absolute ethanol. The catalyst number is A1. Ni accounts for 20% by weight in the catalyst.
[0094] Electroless plating method:
[0095] The process of electroless plating supported NiB amorphous alloy: AgNO with a concent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com