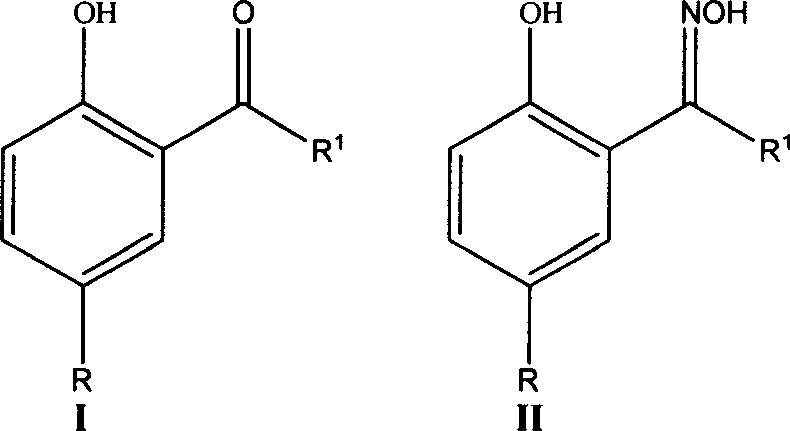
Synthetic method for preparing 2-acyl 4-allcyl phenol
A technology for the synthesis of alkylphenols and synthesis methods, which is applied in the field of synthesis of 2-acyl-4-alkylphenols, an important precursor of metal extractants, can solve problems such as unstable process routes, complicated operations, and high product purity, and avoid Difficulty in product purification, overcoming complex operation, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
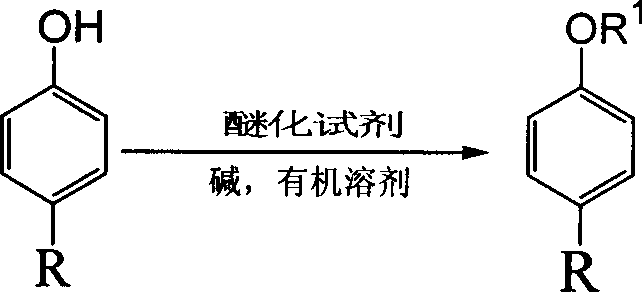
[0029] Embodiment 1: the preparation of 4-hexylphenetole
[0030] 17.8 g of 4-hexylphenol, 16.6 g of anhydrous potassium carbonate, and 100 mL of DMF were sequentially added to a 250 mL three-necked flask. Subsequently, 25.1 g of ethyl iodide was slowly added dropwise into the reaction system, vigorously stirred, and the reaction temperature was controlled at room temperature during the dropwise addition. After the dropwise addition, raise the temperature of the system to 60-70°C, continue to stir for 3 hours, cool to room temperature, pour the reaction solution into 400mL of water, extract the aqueous phase with ethyl acetate, combine the organic phases, and dry with anhydrous magnesium sulfate , to obtain a light yellow liquid after filtration, evaporate the solvent, and carry out vacuum distillation to obtain 19.6 grams of product (b.p.85-90°C / 1mmHg), 1 H NMR (CDCl 3 , 300MHz) δ7.10-7.03 (m, 2H), 6.99-6.89 (m, 2H), 3.82 (q, J=1.1Hz, 2H), 2.11-0.76 (m, 16H) ...
Embodiment 2
[0031] Embodiment 2: Preparation of (4-nonylphenyl) benzyl ether
[0032] 26.2 g of 4-nonylphenol, 11.9 g of pyridine, and 100 mL of dichloroethane were sequentially added to a 250 mL three-necked flask. Subsequently, 16.4 g of benzyl chloride was slowly added dropwise to the reaction system, vigorously stirred, and the reaction temperature was controlled at room temperature during the dropwise addition. After the dropwise addition, the system was heated to reflux, continued to stir for 3 hours, cooled to room temperature, poured the reaction solution into 400 mL of water, extracted the aqueous phase with ethyl acetate, combined the organic phases, dried with anhydrous magnesium sulfate, and filtered Obtain light yellow liquid, distill off solvent, carry out vacuum distillation to obtain product 28.5 grams (b.p.150-153 ℃ / 1mmHg), 1 H NMR (CDCl 3 , 300MHz) δ7.43-6.87 (m, 9H), 4.45 (s, 2H), 2.03-0.61 (m, 19H) ppm; the yield was 92%.
Embodiment 3
[0033] Embodiment 3: Preparation of (4-nonylphenyl) allyl ether
[0034] Into a 250 mL three-necked flask, 22.0 g of 4-nonylphenol, 20.7 g of anhydrous potassium carbonate, and 100 mL of methanol were successively added. Then 15.7 g of allyl bromide was slowly added dropwise to the reaction system and stirred vigorously. The reaction temperature was controlled at room temperature during the dropwise addition. to room temperature, pour the reaction liquid into 600 mL of water, then use ethyl acetate to extract the aqueous phase, combine the organic phases, dry using anhydrous magnesium sulfate, obtain a light yellow liquid after filtration, evaporate the solvent, and carry out vacuum distillation to obtain 24.2 g of the product (b.p.115-118℃ / 1mmHg), 1 H NMR (CDCl 3 , 300MHz) δ7.20-7.14(m, 2H), 7.05-6.97(m, 2H), 5.64-5.52(m, 1H); 5.33-5.03(m, 2H), 4.35-4.25(d×t, 2H ), 2.06-0.57 (m, 19H) ppm; the yield was 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com