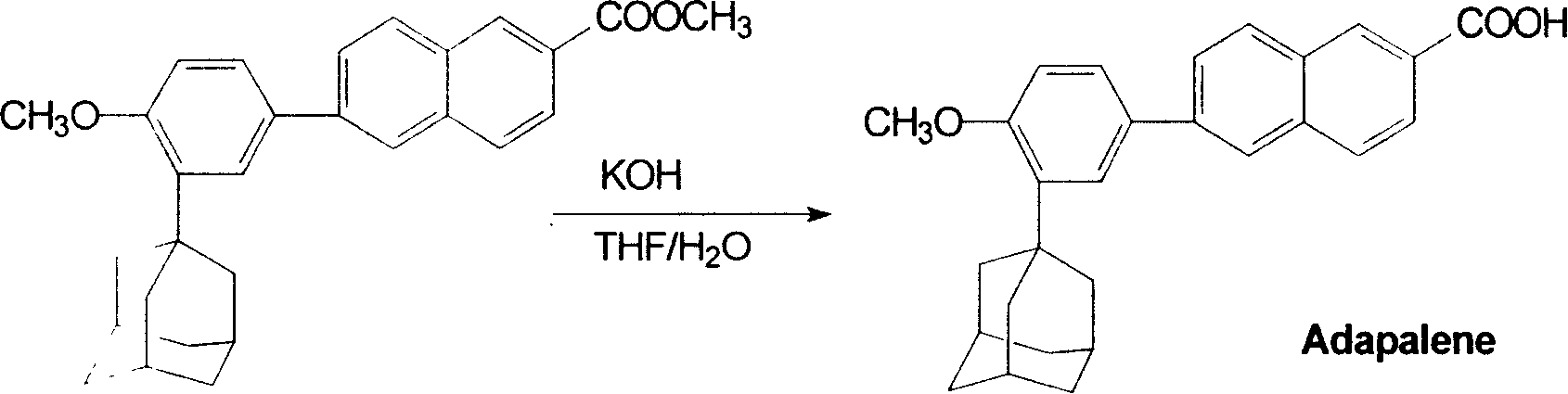
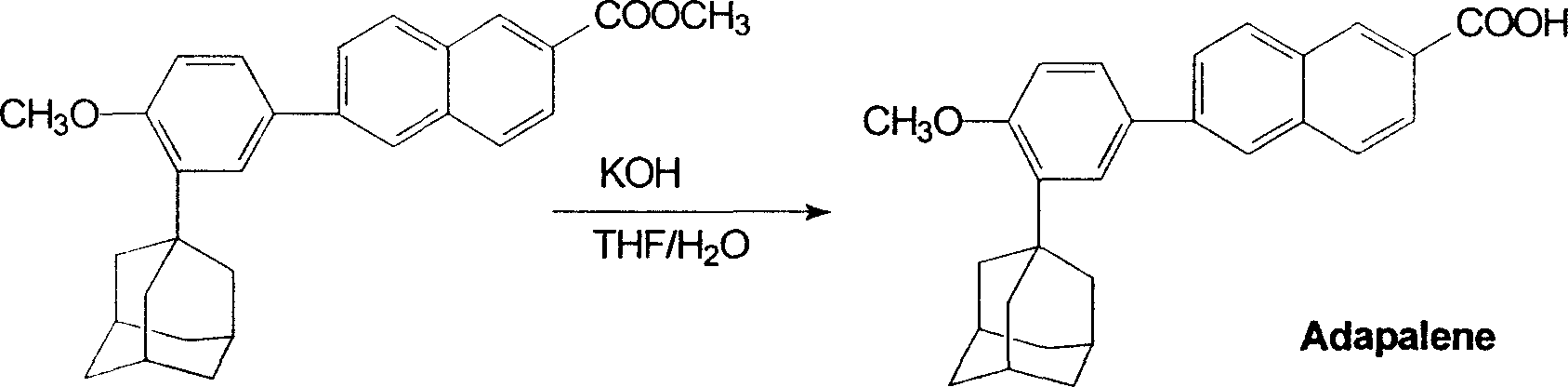
Method for preparing Adapalene
An equation and reaction technology, applied in the preparation of carboxylate/lactone, organic chemistry, etc., can solve the problems of resource consumption, serious three wastes, and high cost, and achieve the effect of reducing costs, reducing resource consumption, and reducing three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0011] In a 250ml four-necked flask, add 2.2g of 6-[3-(1-adamantyl)-4-methoxyphenyl]-2 naphthoic acid methyl ester and 50ml of tetrahydrofuran, methanol, ethanol or acetone, heat Make it dissolve, then add the prepared potassium hydroxide solution (50ml water and 2gKOH), reflux reaction for 2 hours (TLC tracking reaction), after the reaction is complete, concentrate to remove tetrahydrofuran, and the temperature is raised to 80°C to complete precipitation. Add 100 ml of water to the residue, filter to obtain a solid, wash with water until neutral, add the solid to 100 ml of water, acidify to pH 1 with concentrated hydrochloric acid, stir for 1.5 hours, and filter to obtain a crude product. The crude product was recrystallized from a mixed solvent of ethyl acetate and tetrahydrofuran to obtain 1.9 g of the product with a yield of 89.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com