Organic electroluminescent material and its application
A technology selected from aromatic groups, applied in the field of organic electroluminescence display, can solve the problems of device performance attenuation, low luminous brightness and efficiency, and difficulty in actual production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
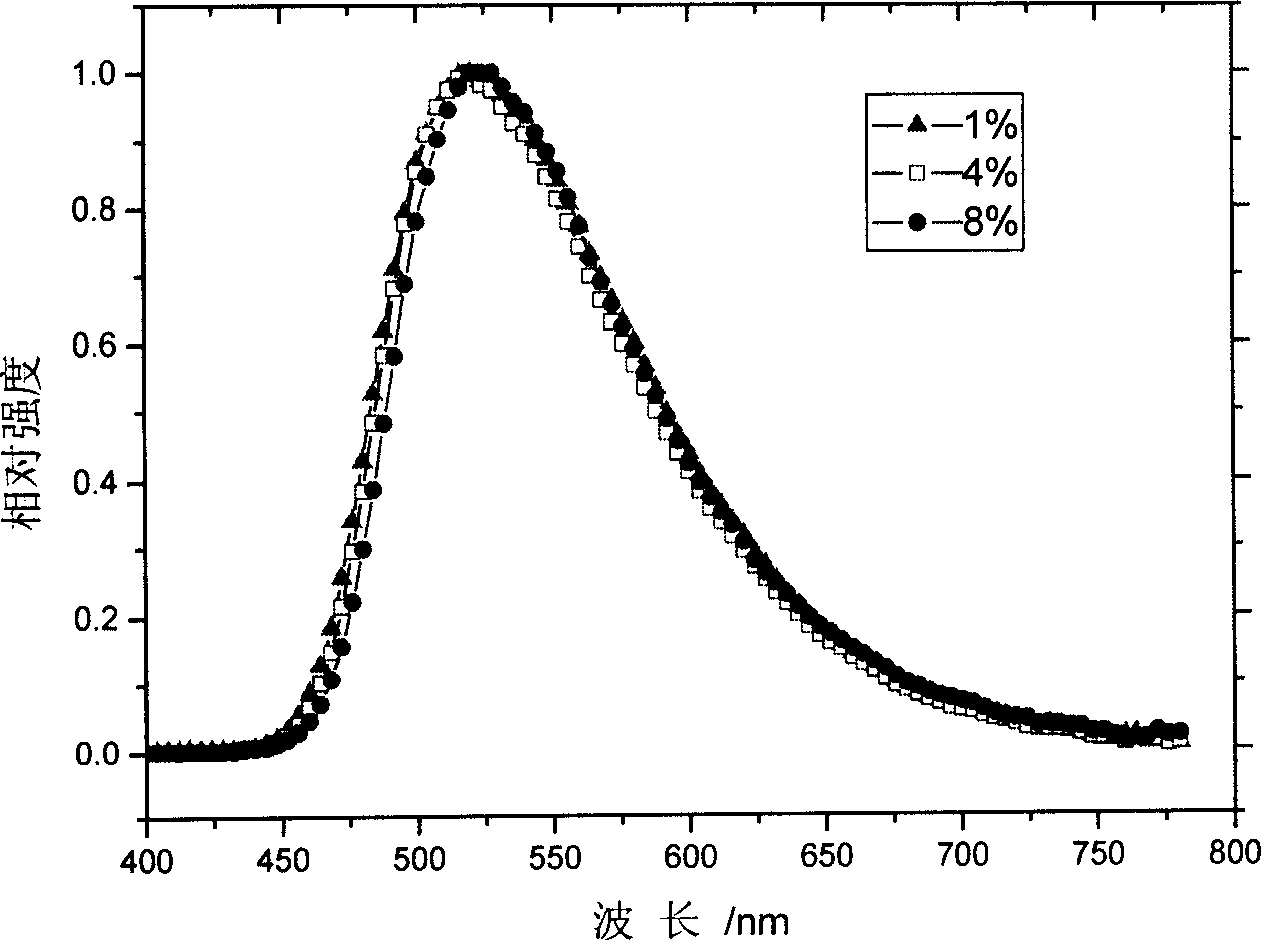
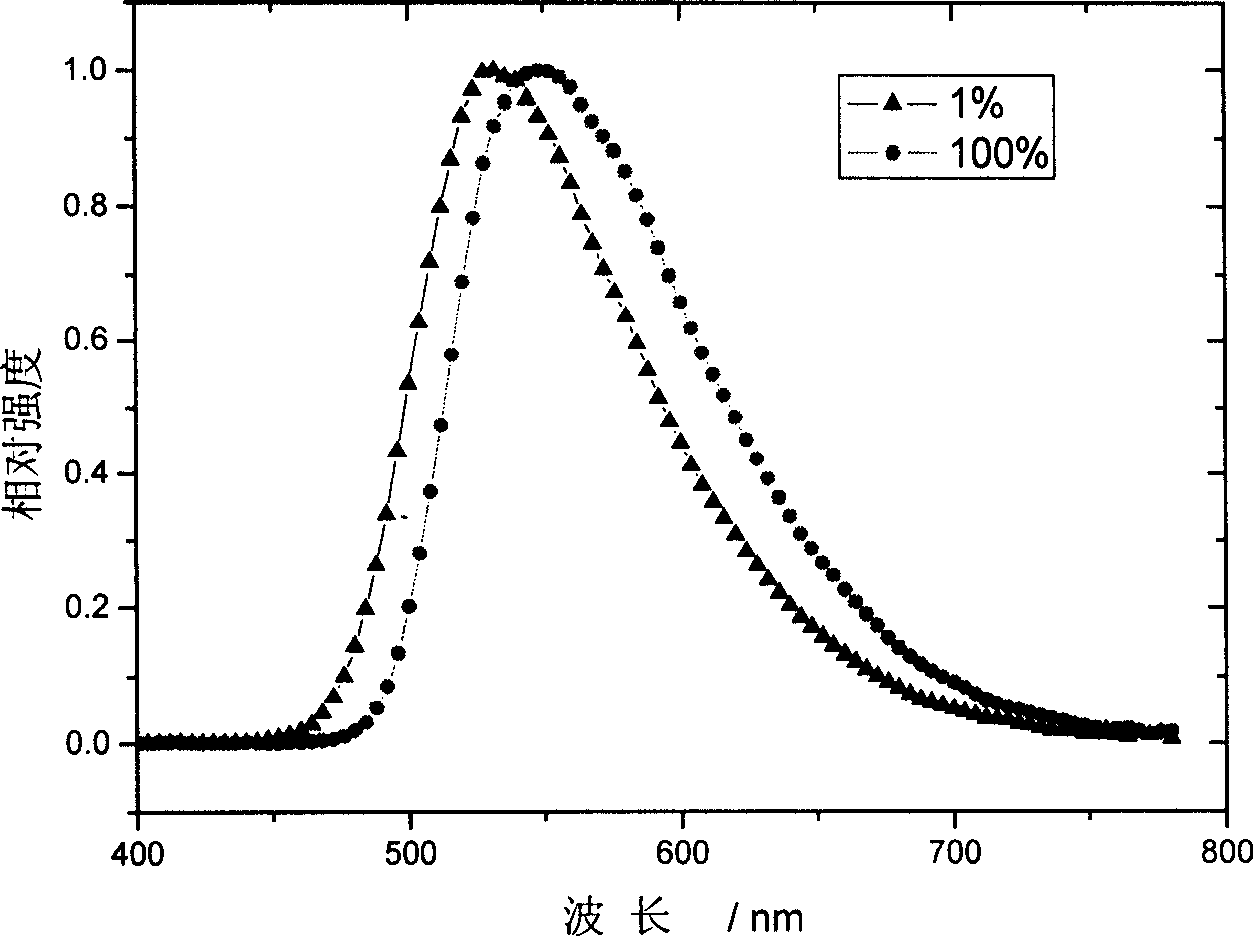
Image
Examples
Embodiment approach
[0030] Preferred embodiment: the compounds of the present invention are all prepared by reacting aryl or heterocyclic aryl boronic acid with the precursor of dibromobenzoxadiazole compounds.
[0031] Preparation of raw material arylboronic acid:
[0032] Most of the aryl boronic acid raw materials used in the present invention are purchased from Bailingwei Company, and the parent body of dibromobenzoxadiazole and part of aryl boronic acid are synthesized according to the following method.
[0033] Synthesis of 4-biphenylboronic acid:
[0034] Reaction formula:
[0035]
[0036] Process: In a 100ml three-neck flask equipped with magnetic stirring, reflux condenser and nitrogen protection device, dissolve 5.83 grams of 4-bromobiphenyl (0.025mol) in 20mlTHF, add 0.85 grams of magnesium chips (0.035mol), 0.5ml bromoethane, warm to start the reaction, reflux for 2 hours. Cool 3.12 grams (3.25ml, 0.03mol) of trimethyl borate to -5°C to -10°C with an ice-salt bath, slowly add 4...
Embodiment 1
[0071] Example 1 4,7-diphenyl-2,1,3-benzoxadiazole (compound i-4)
[0072] Reaction formula:
[0073]
[0074] process:
[0075] In a 100mL three-necked flask equipped with a magnetic stirrer, a condensing reflux device and a nitrogen protection device, 4,7-dibromobenzoxadiazole (1.50 g, 5.2 mmol), anhydrous potassium carbonate (3.59 gram, 26.0mmol), 4-biphenylboronic acid (2.52 gram, 12.5mmol), two (triphenylphosphine) palladium dichloride (0.70 gram, 1.0mmol) and by toluene, ethanol and water (volume ratio is 3: 3:2) of the mixed solution consisting of 65 mL, heated to reflux under the protection of nitrogen, and reacted for 24 hours. Remove from heat and cool to room temperature.
[0076] Pour the reaction solution into 50 mL of water, filter under reduced pressure, rinse the filter cake with water and ethyl acetate in sequence, dry the solid, and recrystallize with toluene to obtain a yellow product.
[0077] Product MS (m / e): 424.2; Elemental analysis (C 30 h 20 ...
Embodiment 2
[0078] Example 2 4,7-bis(1-naphthyl)-2,1,3-benzoxadiazole (compound i-5)
[0079] Reaction formula:
[0080]
[0081] The process is the same as in Example 1, except that the raw material is replaced with 1-naphthylboronic acid to obtain a yellow product.
[0082] Product MS (m / e): 372.4; Elemental analysis (C 26 h 16 N 2 O): theoretical value C: 83.85, H: 4.33, N: 7.52; measured value C: 83.82, H: 4.30, N: 7.47.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com