Pesticide uses of compound 4-hydroxy sesquiterpene propyl ester
A technology of semiterpene propyl esters and compounds, applied in the fields of application, organic chemistry, animal repellent, etc., can solve the problems of not specifying the use of compounds, and achieve the effects of saving manpower, long validity period, and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation and contact activity determination of Daphne chamaejasme extract
[0031] Chamaejasma chamaejasma is collected from Ruoergai Grassland in Sichuan Province. The collected fresh chamaejasma roots are washed, dried, and pulverized with a grinder to obtain fluffy chamaejasma powder.
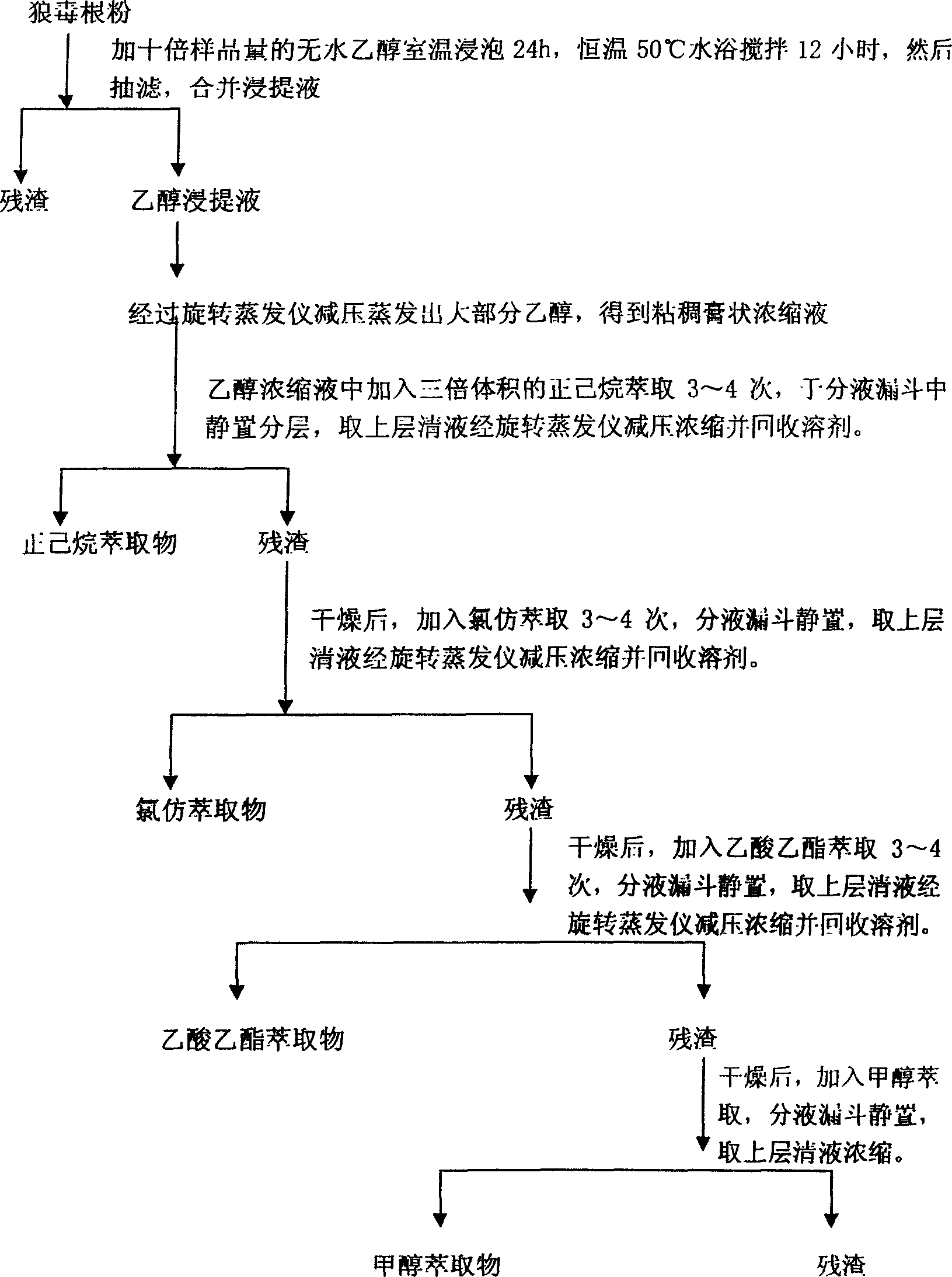
[0032] Weigh a certain amount of chamaejasma root dry powder, add 10 times the mass of absolute ethanol to soak, extract and concentrate, and then use different polar solvents n-hexane, chloroform, ethyl acetate, methanol for graded extraction to obtain different polar extracts , repeated 3 times until the extraction was complete, and the same extracts were combined.
[0033] According to the method introduced by Zhang Zongbing, the antifeedant activity and contact activity were used to measure the toxicity: first, the cabbage caterpillars were collected in the vegetable fields in the suburbs where no pesticides had been applied, and healthy third-instar individuals with ...
Embodiment 2
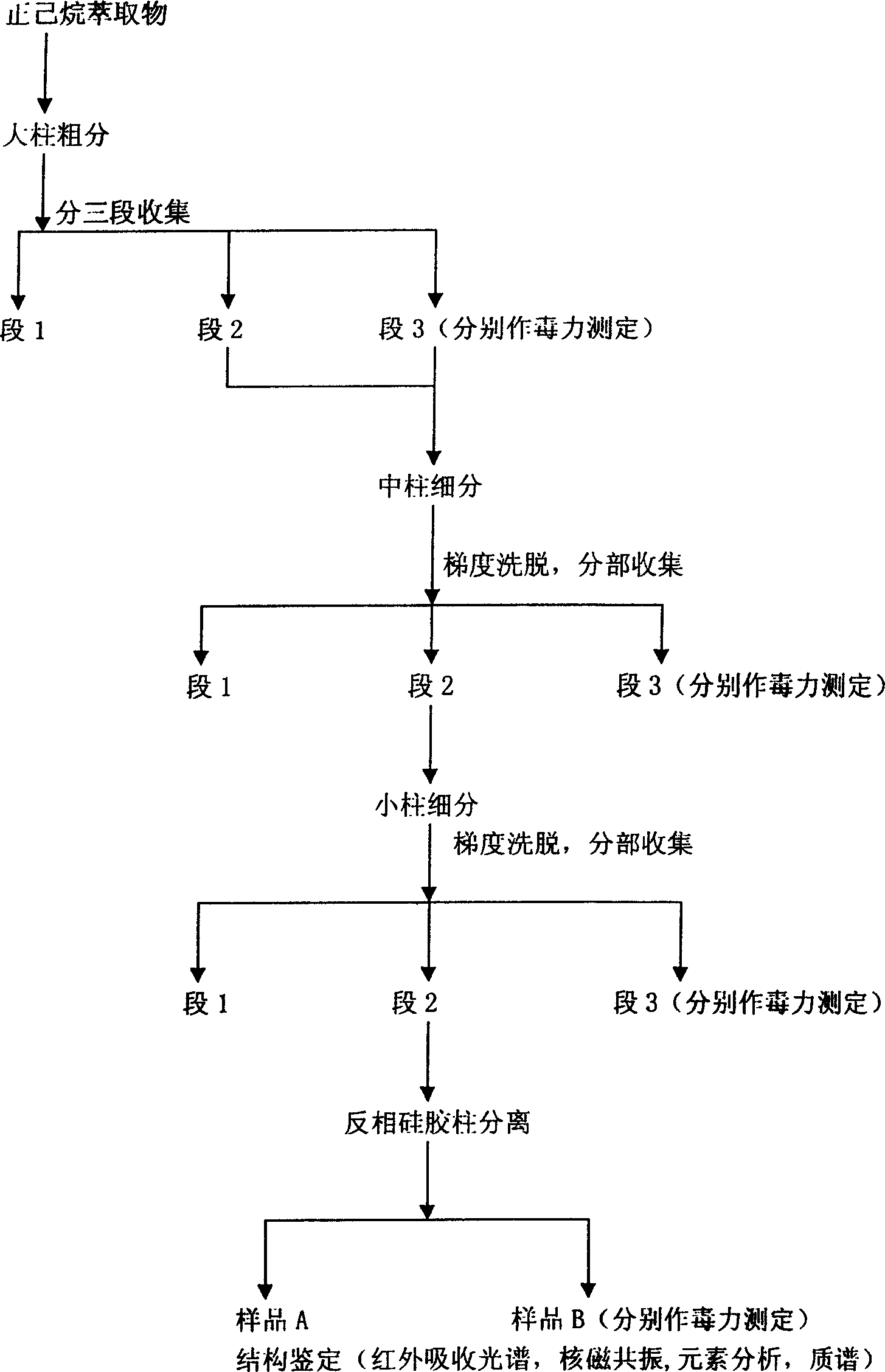
[0042] Embodiment 2 Chromatographic separation and purification of Daphne chamaejasme insecticide active substance
[0043] Thin-layer chromatography silica gel GF254 was used as the stationary phase of adsorption chromatography, and plates were plated on a thin-layer automatic plate plater according to conventional methods. Sheet size: 5×10(cm), silica gel thickness: 0.25mm. Put the laid boards into an oven at 105°C for half an hour, and store them in a desiccator after cooling for later use. Use a spotting capillary to spot a sample, use n-hexane, chloroform, ethyl acetate, dichloromethane, methanol and other solvents with different compositions and different proportions of mixed solvents as developing agents, and after developing, irradiate with 254nm ultraviolet analysis lamp and iodine Three methods of powder vapor and sulfuric acid-ethanol color development were combined to detect bands. Choose a solvent with good separation effect and no tailing for reference in colum...
Embodiment 3
[0062] Embodiment 3 active substance structure identification
[0063] Compound A
[0064] Compound A is a light yellow oily substance, easily soluble in organic solvents such as petroleum ether, chloroform, ethyl acetate, etc., and shows blue-purple spots on the thin layer of silica gel GF254 under the ultraviolet analysis lamp. Spectral data analysis is as follows:
[0065] Uv λmax (methanol) nm: 283.0, 309.0 (weak). The weak peak at 309.0nm of the ultraviolet absorption peak is n→π of (carbonyl) * Transition, UV absorption peak 283.0 is π→π * transition.
[0066] IR νmax cm -1 : 3400.81 (hydroxyl, blunt peak), 3027.84 (benzene ring hydrogen), 2930.28, 2858.62 (saturated hydrogen), 1682.26 (carbonyl), 1598.67, 1581.49, 1496.20, 1450.86, 753.90, 699.65 (monosubstituted benzene ring).
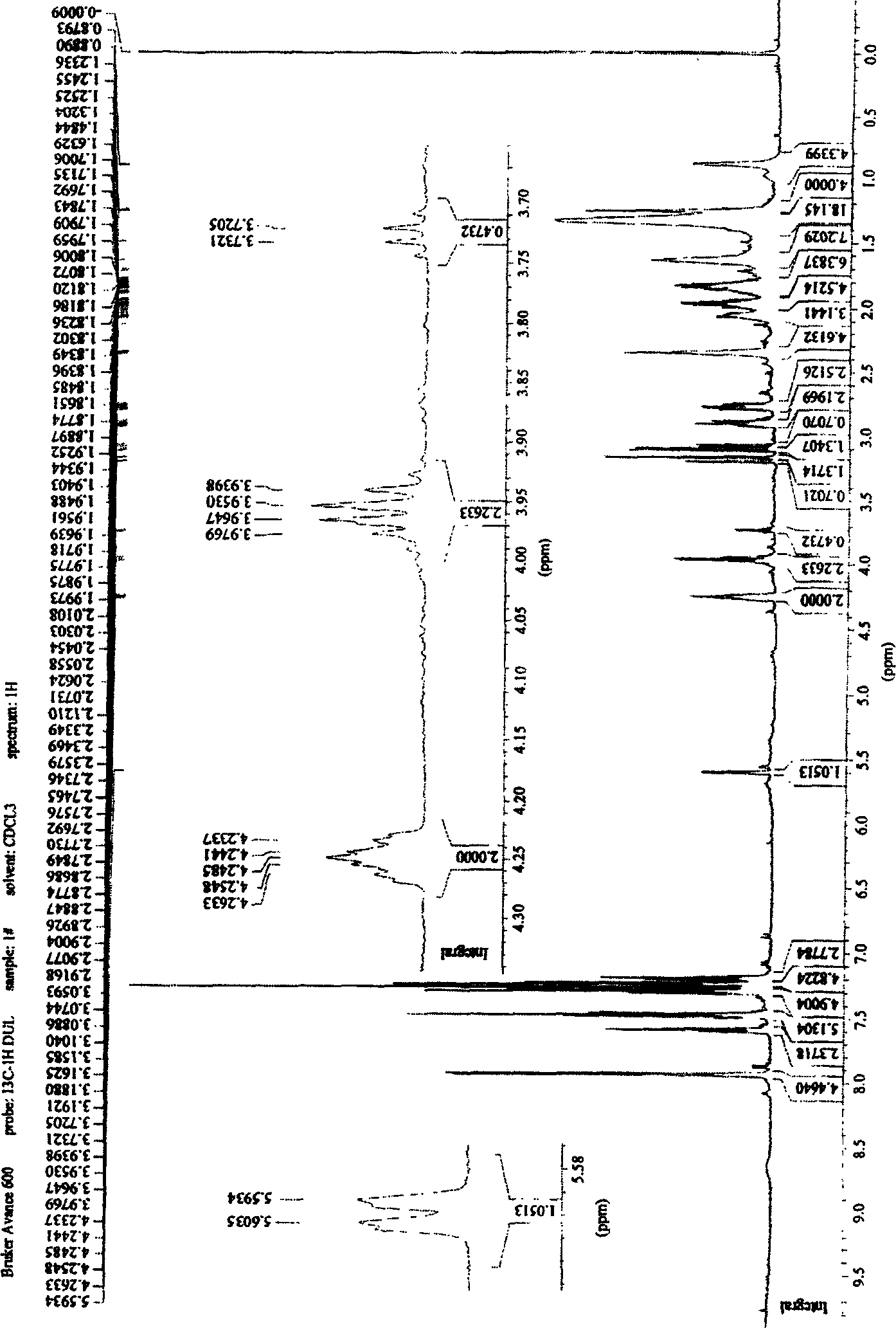
[0067] 1 H-NMR (CDCl 3 , 600Hz) δppm: 1.90 (2H, dm) for 4-digit H, δ2.83 (2H, dm), for 5-digit H, δ3.13 (2H, M), for 2-digit H, δ3.95 (1H, m) is hydroxyl H, δ4.25 (1H, m) is 3-position...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com