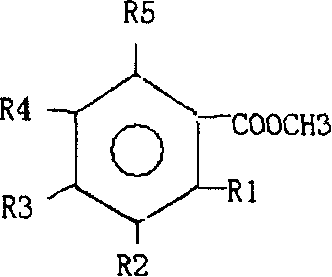
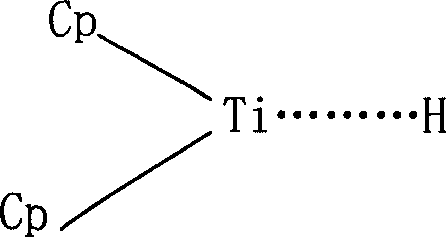
Process for selective hydrogenation of styrene-conjugated diene block polymer
A conjugated diene block and block polymer technology, which is applied in the field of selective hydrogenation of styrene-conjugated diene block polymers, can solve the problem of complex catalyst preparation, difficult hydrogenation degree, good process reproducibility, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The following examples will further illustrate the present invention, but do not thereby limit the scope of the present invention.
[0025] In a 5L autoclave purified by pure nitrogen, add 3500ml of cyclohexane, 57ml of styrene, 0.159mmol of butyllithium, and the calculated amount of a microstructure regulator (tetrahydrofuran or ethylene glycol diethyl ether is used as the microstructure regulator) at 40 After 30 minutes of reaction at ℃~60℃, 395ml of butadiene was added to react for 35 minutes, and finally 57ml of styrene was added, and the reaction was carried out for 25 minutes to obtain a glue solution of styrene-conjugated diene triblock polymer. Under the protection of pure nitrogen, it was introduced into a nitrogen-purified 5L hydrogenation kettle, and different co-catalysts were added to investigate the influence of different co-catalysts on the hydrogenation reaction and the color of the SEBS product. The conditions of the hydrogenation reaction were: the main...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com