Combination therapy for glycaemic control
A technology for blood sugar control and use, which is applied in the therapeutic field of blood sugar control, and can solve the problems of low efficiency, time efficacy, reduction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0088] - No activity against non-DP IV and non-DP IV-like enzymes, such as DP I, prolyl oligopeptidase, aminoacyl proline dipeptidase (see Example 12);
[0089] - high stability in vitro in isolated human plasma (see Example 13);
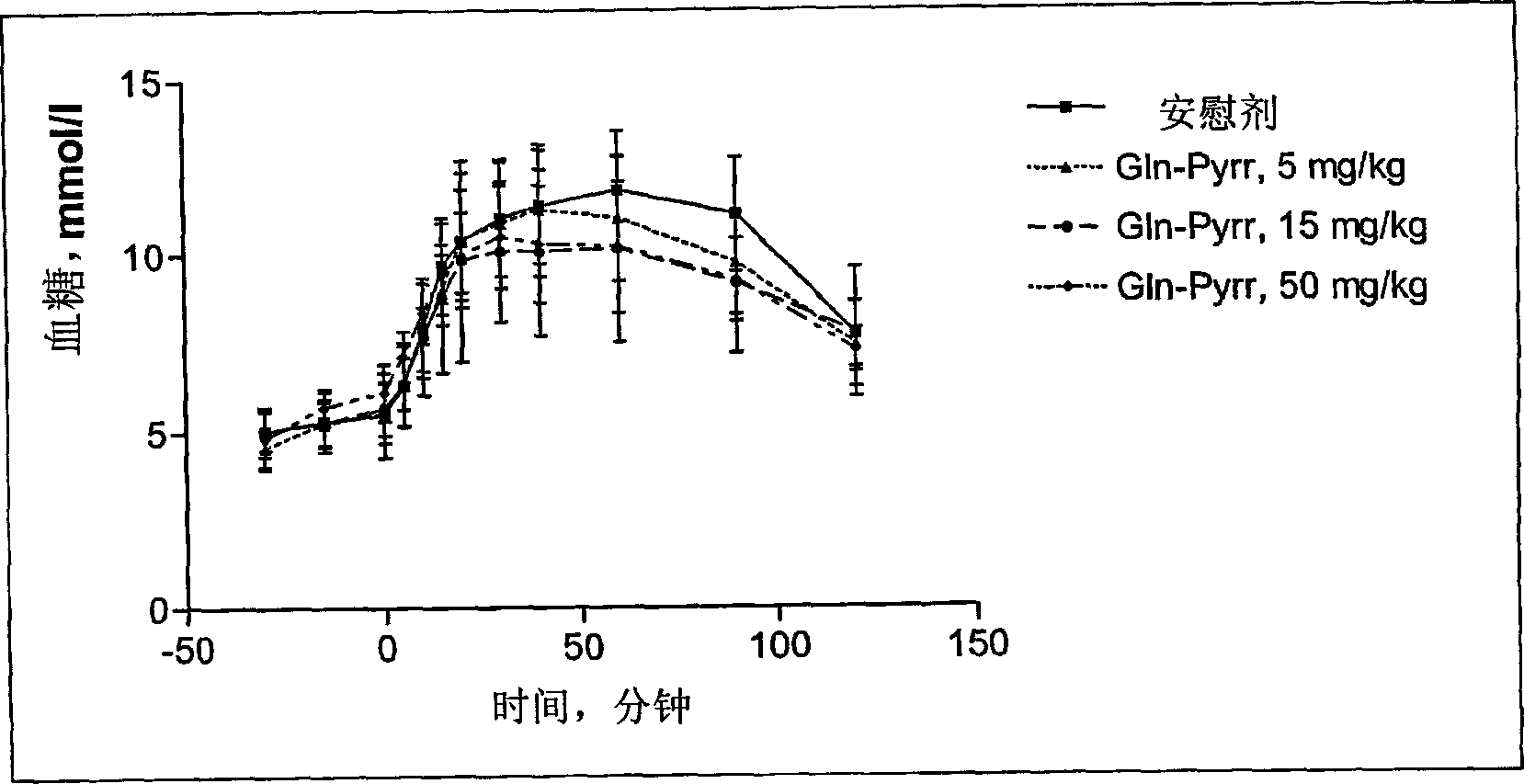
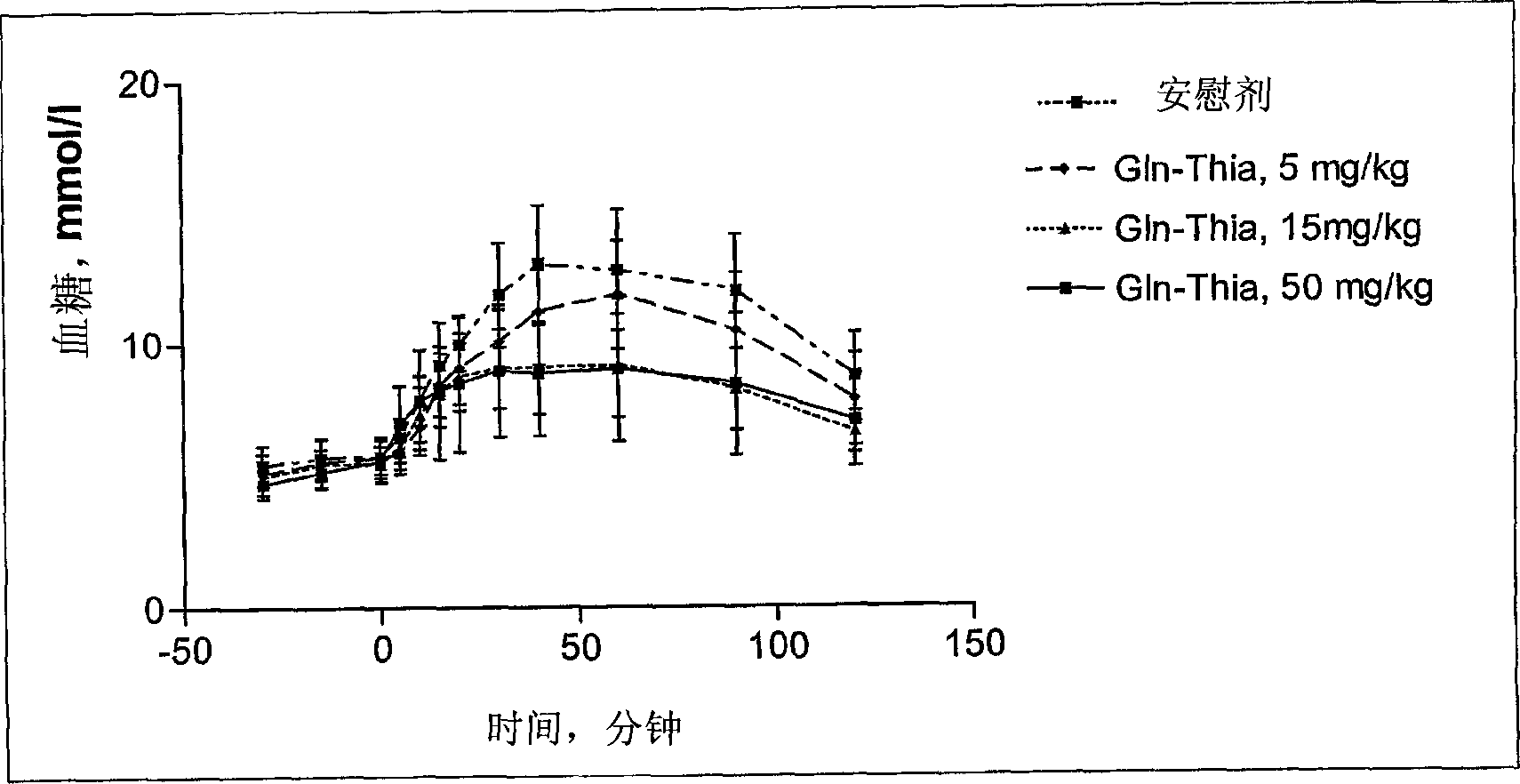
[0090] - a novel and controllable mechanism for the partial inactivation / metabolism of glutamine to the corresponding pyroglutamyl compound in vivo, resulting in a shorter half-life than other DP IV inhibitors (see Example 8); and
[0091] - Predicted in vivo liver-independent half-life.
[0092] Pharmaceutically acceptable salts of compounds of formula (I) include acid addition salts, ie wherein the basic side chain of the amino acid is protonated with an inorganic or organic acid. Representative organic or inorganic acids include hydrochloric, hydrobromic, perchloric, sulfuric, nitric, phosphoric, acetic, propionic, glycolic, lactic, succinic, maleic, fumaric, malic, tartaric, Citric acid, benzoic acid, mandelic acid, methanesulfonic acid, iseth...
Embodiment 1
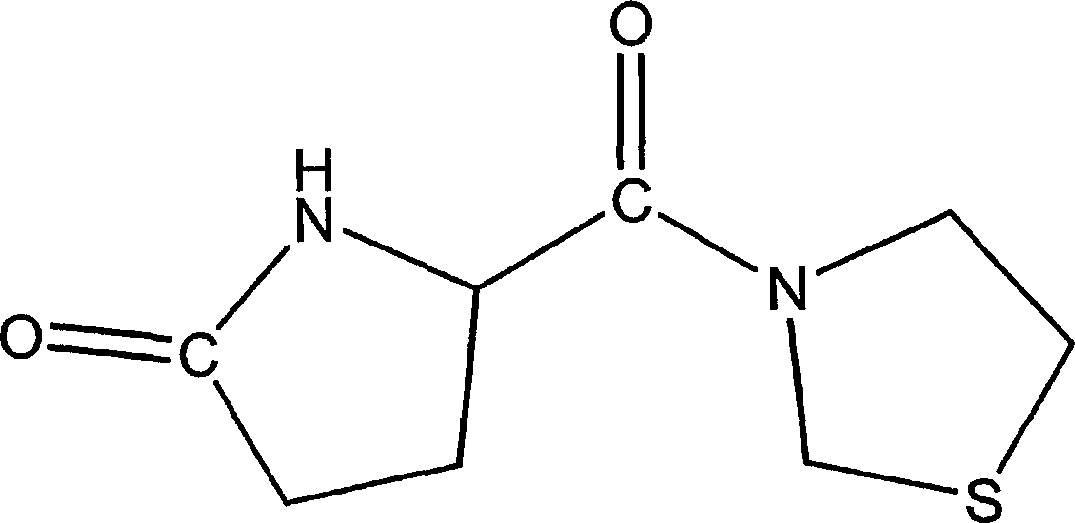
[0178] Embodiment 1: the synthesis of glutaminyl pyrrolidine free base
[0179] N-Benzyloxycarbonylglutamine (2.02 g, 7.21 mmol) was dissolved in 35 mL THF and cooled to -15°C. CAIBE (isobutyl chloroformate) (0.937 mL, 7.21 mmol) and 4-methylmorpholine (0.795 mL, 7.21 mmol) were added and the solution was stirred for 15 minutes. The formed mixed anhydride was detected by TLC (eluent: chloroform / methanol: 9 / 1). After warming to -10°C, pyrrolidine (0.596 mL, 7.21 mmol) was added. The mixture was brought to room temperature and stirred overnight. The precipitate formed was filtered off and the solvent was evaporated. The resulting oil was taken up in ethyl acetate (20 mL) and washed with saturated sodium bisulfate solution followed by saturated sodium bicarbonate solution, water and brine. The organic layer was separated, dried and evaporated. The purity of the obtained product was checked by TLC (eluent: chloroform / methanol: 9 / 1). Yield: 1.18 g. This product was dissolved...
Embodiment 2
[0180] Embodiment 2: the synthesis of glutamyl thiazolidine hydrochloride
[0181] N-tert-butoxycarbonylglutamine (2.0 g, 8.12 mmol) was dissolved in THF (5 mL), cooled to -15°C. CAIBE (isobutyl chloroformate) (1.06 mL, 8.12 mmol) and 4-methylmorpholine (0.895 mL, 8.12 mmol) were added and the solution was stirred for 15 minutes. The formed mixed anhydride was detected by TLC (eluent: chloroform / methanol: 9 / 1). After warming to -10°C, another equivalent of 4-methylmorpholine (0.895 mL, 8.12 mmol) and thiazolidine hydrochloride (1.02 g, 8.12 mmol) were added. The mixture was brought to room temperature and stirred overnight. The precipitate formed was filtered off and the solvent was evaporated. The resulting oil was taken up in chloroform (20 mL) and washed with saturated sodium bisulfate solution followed by saturated sodium bicarbonate solution, water and brine. The organic layer was separated, dried and evaporated. The purity of the obtained product was checked by TLC ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com