Optical-purity meptazinol orits salts, and preparing method
A technology of meprotamol and protamol, which is applied in the fields of organic chemistry, active ingredients of heterocyclic compounds, nervous system diseases, etc., and can solve problems such as the development and research of optically pure meprotamol.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
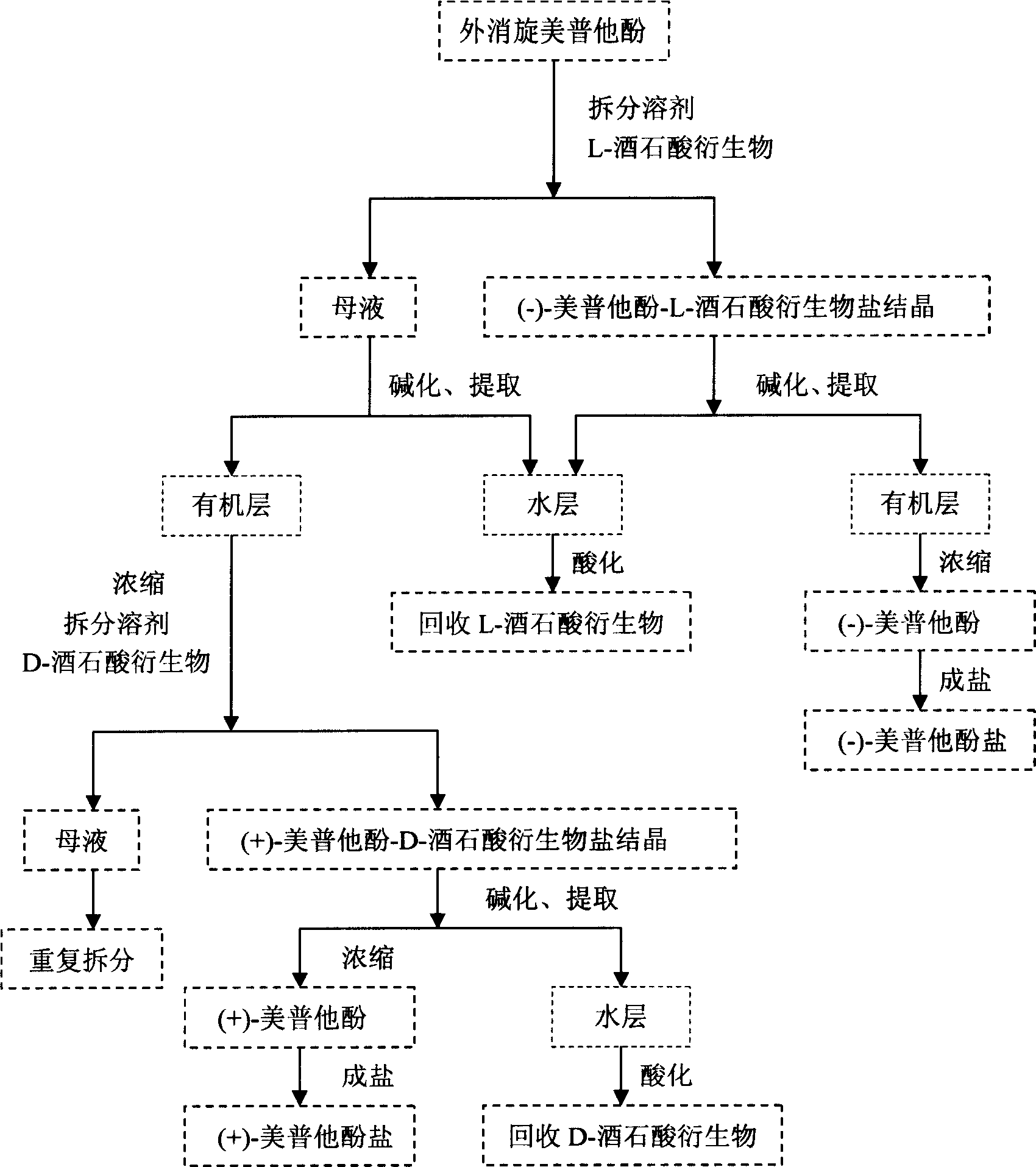
[0023] Example 1 Resolution of Mebutamol with Optically Pure Dibenzoyl Tartaric Acid
[0024] 10 grams of meprotamol racemate (preparable by the method disclosed in US Patent No. 4197239), 300 ml of absolute ethanol, and reflux to dissolve. Add 15.36 g of L-dibenzoyl tartaric acid / 50 ml of absolute ethanol solution dropwise, and add 600 ml of ethyl acetate after reflux for 1 hour, and 11.35 g of white solid is precipitated, and white crystals are obtained after recrystallization from methanol, namely (-)-Mepro Hephenol-L-dibenzoyl tartrate, melting point 171~173℃, [α] D -96.29°(c=0.105, MeOH), the white crystals were basified with sodium carbonate solution, extracted with ether, concentrated to obtain (-)-mebutamol 2.80g, and then salted with hydrogen chloride in ethanol solution to obtain (-) - Mebutamol hydrochloride 2.97g, melting point 214-5°C, [α] D -10.72° (c=0.167, H 2 O), e.e.=99.06%, based on Mebutamol, the total yield is 25.7%.
[0025] Split the mother liquor an...
Embodiment 2
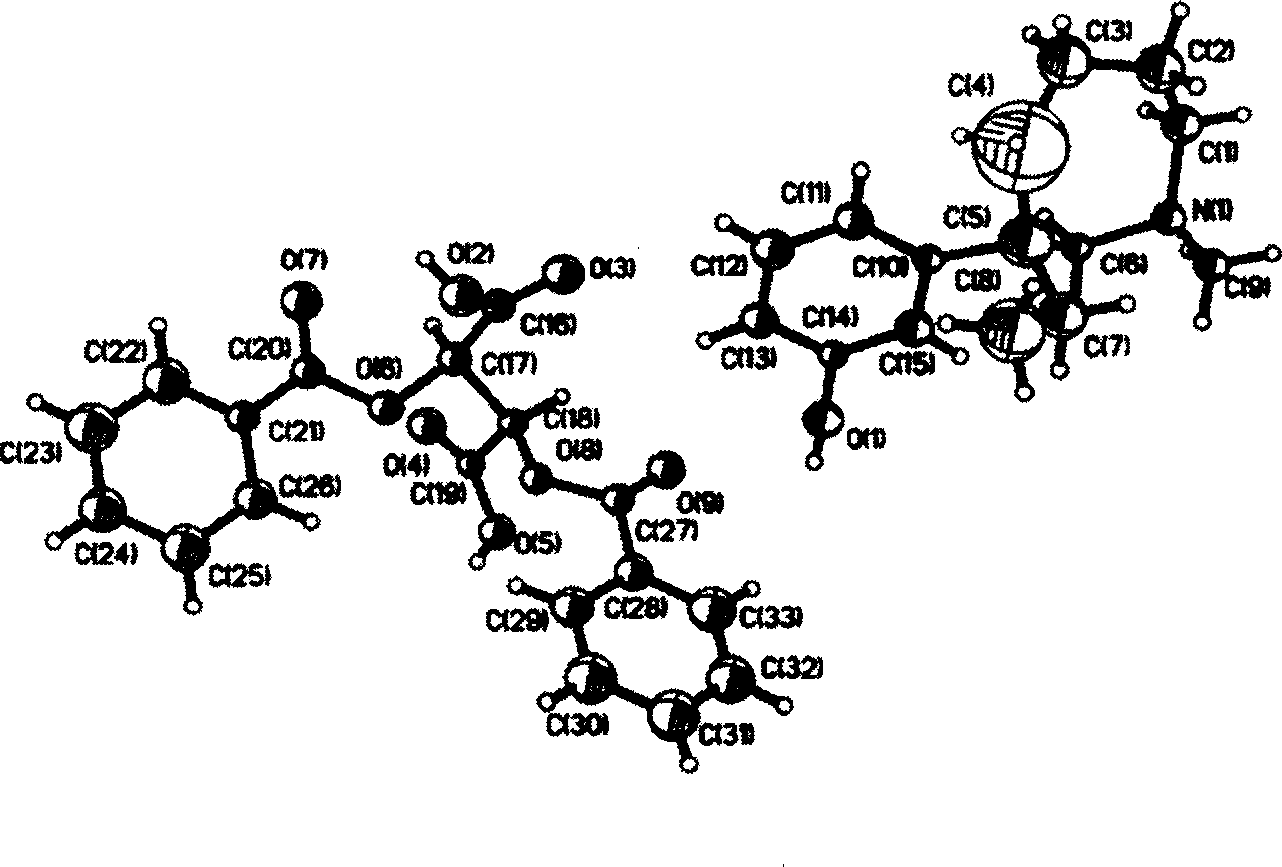
[0027] Example 2 Single crystal X-ray diffraction test of (-)-mabutaol-L-dibenzoyl tartrate
[0028] (1) Instrument model: DIFFACTIS 586 X-ray four-circle diffractometer
[0029] (2) Computer and software package: SHELXS97 and SHELXL97 / 2 programs
[0030] (3) Single crystal preparation:
[0031] The (-)-meprotamol-L-dibenzoyl tartrate obtained in the examples was obtained by recrystallization from methanol.
[0032] (4) Experimental test:
[0033] Select a single crystal of 0.15mm × 0.15mm × 0.10mm, measure the unit cell parameters with the DIFFACTIS 586 X-ray four-circle diffractometer produced by Enraf-Nonius at room temperature, and collect the diffraction data, and use MoKα radiation (λ = 0.071073nm), in the range of 1.72°2σ(I)]. All intensity data are corrected by LP factor, the structure is solved by direct method, and all non-hydrogen atom coordinates are obtained by difference Fourier synthesis method. Deviation factor R=0.0673, R W2=0.1511, S=0.732, the absolute...
Embodiment 3
[0037] The mensuration of embodiment 3 optical purity
[0038] The instrument uses Agilent capillary electrophoresis system. Use 72cm×50μm uncoated quartz capillary, 30mmol / L phosphate buffer solution (pH8.05, containing 0.5% TM-β-CD, acetonitrile 12%), operating voltage 20kV, capillary column temperature 20℃, pressure injection 3kPa ×3s, detection wavelength 200nm. Baseline separation of the enantiomers of mebutamol was achieved under the selected experimental conditions. In the concentration range of 0.01-0.50mg / mL for the two enantiomers, the response of the concentration and the peak area showed a good linear relationship. The RSD of migration time is within 3%, the RSD of peak area is within 10%, and the minimum detection concentration is 0.01mg / mL. The optical purity of dibenzoyl tartrate meprotamol dextrorotary body, di-p-toluoyl tartrate meprotamol dextrorotary body, meprotamol hydrochloride dextrorotary body and left-handed body were 99.63%, 99.63%, respectively. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com