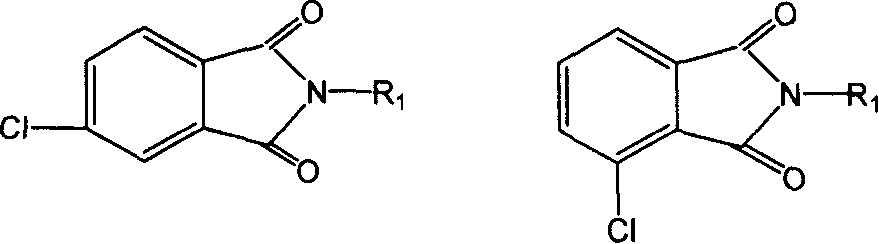Method for preparing diether type tetraacid dianhydride isomer
A technology of tetraacid dianhydride and isomer is applied in the field of preparation of bisether-type tetraacid dianhydride isomer, and can solve the problems of high cost of raw materials, difficult environmental protection and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 0.1mol (11.0 grams) of hydroquinone, 0.216mol (42.25 grams) of N-methyl-4-chlorophthalimide, 0.22mol (30.36 g) anhydrous potassium carbonate and 250 milliliters of dry dimethyl sulfoxide, stir after nitrogen deoxygenation, heat to 90 ° C, and stir at this temperature for 5 hours, after the reactant is cooled to room temperature, pour it into The precipitate was filtered out in 500 ml of water, washed with hot water and hot ethanol respectively, and dried to obtain 40.7 g of the product 4,4'-hydroquinone tetraimide with a yield of 95%. 21.4 g of the obtained product was heated and refluxed in 140 ml of 10% aqueous sodium hydroxide solution for 5 hours, and the aqueous solution was acidified to PH=3 with hydrochloric acid, and a white solid was precipitated, filtered, washed with water, and dried to obtain tetraacid, which was dehydrated to obtain 19.3 gram of 4,4'-hydroquinone-type tetraacid dianhydride, and the yield was 96%.
Embodiment 2
[0028] Add 0.1mol (22.83 grams) bisphenol A, 0.22mol (43.03 grams) N-methyl-4-chlorophthalimide, 0.24mol (33.12 grams) anhydrous potassium carbonate in a 500 ml three-necked flask and 170 ml of dry dimethylformamide, stirred with nitrogen to remove oxygen, heated to 130 ° C, and stirred at this temperature for 10 hours, after the reactant was cooled to room temperature, it was poured into 400 ml of water, filtered out The precipitate was washed with hot water and hot ethanol respectively, and dried to obtain 50.8 g of the product 4,4'-bisphenol A tetraimide with a yield of 93%. The resulting 27.3 g product was heated to reflux in 80 ml of 20% sodium hydroxide aqueous solution for 5 hours, and the aqueous solution was acidified to PH=2 with hydrochloric acid. After boiling for 5 minutes, the white solid was cooled and precipitated, filtered, washed with water, and then dried to obtain the tetraacid. The tetraacid was dehydrated with acetic anhydride to obtain 23.7 g of 4,4'-bis...
Embodiment 3
[0030] Add 0.1mol (11.0 grams) of resorcinol, 0.18mol (46.38 grams) of N-phenyl-4-chlorophthalimide, 0.20mol (27.6 grams) of anhydrous Potassium carbonate and 140 ml of dry N-methylpyrrolidone, after nitrogen deoxygenation, stirred and heated to 180 ° C, and continued to react at this temperature for 3 hours, cooled and poured into 400 ml of water, a white precipitate was precipitated, and the solid was collected by filtration , washed the filter cake twice with hot water and hot ethanol respectively, and dried to obtain 51.4 grams of 4,4'-resorcinol-type tetraimide, with a yield of 94%. The obtained 27.6 g of tetraimide and 70 ml of 30% sodium hydroxide aqueous solution were heated and refluxed for 5 hours, the aqueous solution was acidified to PH=1 with hydrochloric acid, a white solid was precipitated, filtered, washed with water and then dried to obtain the tetra-acid, and the tetra-acid was washed with ethyl alcohol. The acid anhydride was dehydrated to obtain 18.1 g of 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com