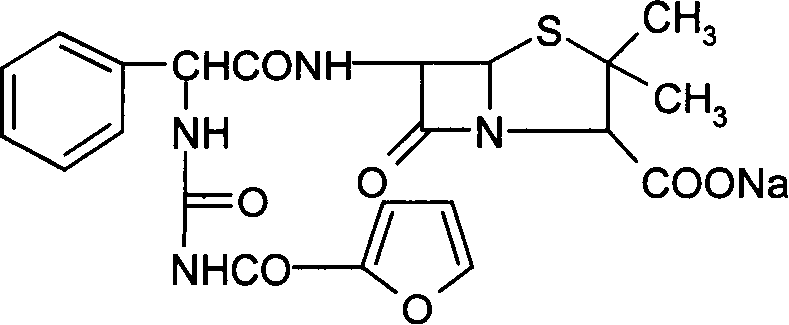
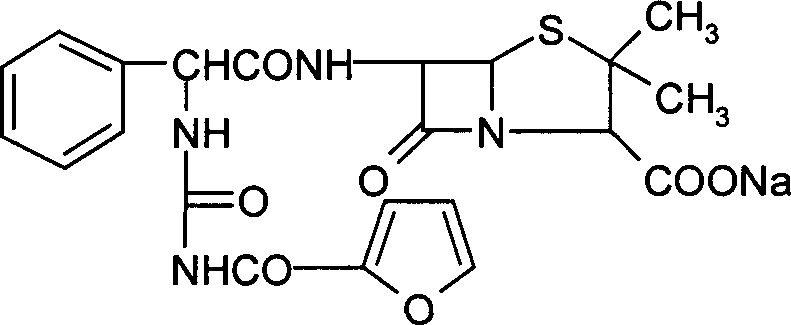
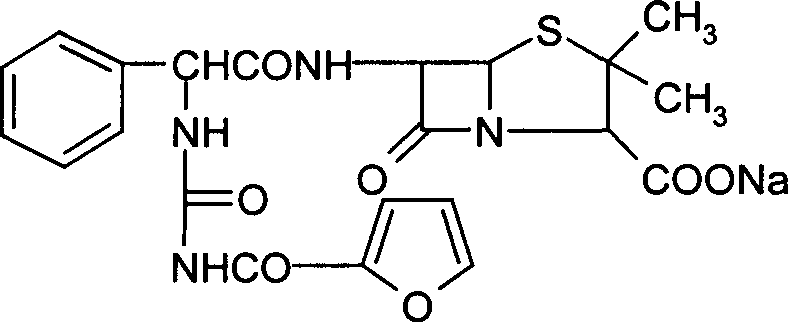
Furo urea penicillin sodium crystal and its synthesis
A technology of furacillin sodium and ampicillin acid, which is applied in the field of furacillin sodium crystal and its synthesis, can solve the problems of poor product stability, long synthesis route, many reaction and processing steps, etc., and achieve the reduction of solvent consumption, The effect of short production cycle and simple route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Add 32 g of tetrahydrofuran into a 250 ml four-neck flask, stir and add 10 g of anhydrous ampicillin acid. Since anhydrous ampicillin acid is insoluble in tetrahydrofuran, the reaction solution is in a suspension state. The reaction system was cooled to -5°C to 10°C while stirring, and 4.3 g of 2-furanoyl isocyanate was added dropwise. The reaction solution gradually became clear to obtain a furosemide penicillin acid solution. Maintain the temperature at -5°C to 10°C, then add 5% sodium methoxide in isopropanol solution (every 100g of isopropanol contains 5 grams of sodium methoxide), until the pH of the reaction solution is 6.8, slowly in the reaction system A white crystalline substance, that is, crystals of furacillin sodium, was precipitated. After filtering, the filter cake was washed twice with isopropanol, sucked dry and dried below 60° C. to obtain 13.25 g of fururabenicillin sodium crystals with a yield of 91.03%. As detected by high-pressure liquid chromato...
Embodiment 2
[0071] Add 40 g of tetrahydrofuran into a 250 ml four-neck flask, stir and add 10 g of anhydrous ampicillin acid. Since anhydrous ampicillin acid is insoluble in tetrahydrofuran, the reaction solution is in a suspension state. While stirring, the reaction system was cooled to -5°C to 10°C, and 4.5 g of 2-furanoyl isocyanate was added dropwise. The reaction solution gradually became clear to obtain a furosemide penicillin acid solution. Then add 52 grams of isopropanol, maintain the temperature at -5°C to 5°C, then add 2% sodium methylate in isopropanol (every 100 grams of isopropanol contains 2 grams of sodium methylate), until the pH of the reaction solution was 6.8, and white crystals slowly precipitated out in the reaction system, i.e. fururabenicillin sodium crystals. After filtering, the filter cake was washed twice with isopropanol, sucked dry and dried at 50° C. to obtain 13.5 g of fururabenicillin sodium crystals with a yield of 93.0%. High pressure liquid chromatogr...
Embodiment 3
[0081] Add 40 g of tetrahydrofuran into a 250 ml four-neck flask, stir and add 10 g of anhydrous ampicillin acid. Since anhydrous ampicillin acid is insoluble in tetrahydrofuran, the reaction solution is in a suspension state. The reaction system was cooled to -5°C to 0°C while stirring, and 4.3 g of 2-furanoyl isocyanate was added dropwise. The reaction solution gradually became clear to obtain a furosemide penicillin acid solution. The reaction solution maintains a temperature of -5°C to 0°C, then adds 5% sodium methylate in dehydrated alcohol (every 100 grams of dehydrated ethanol contains 5 grams of sodium methoxide), until the pH of the reaction solution is 6.8, the reaction solution is carried out After the ethanol was removed under reduced pressure, the solid precipitated out, then dissolved with 95% ethanol, and 70 grams of isopropanol was used to separate out the crystals of furosecillin sodium, filtered and dried to obtain 13.3 grams of furosecillin sodium crystals, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com