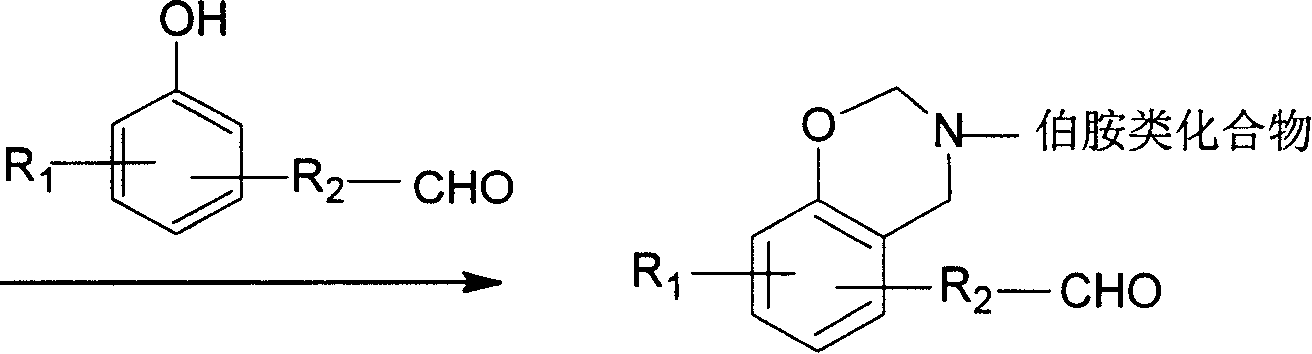
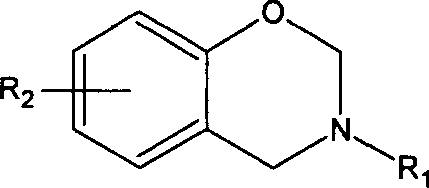
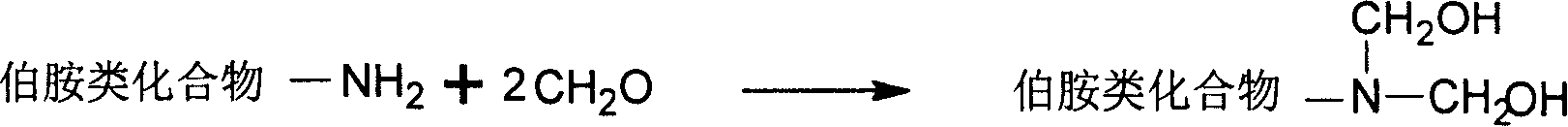Benzo oxaxine intermediate containing aldehyde group and its preparation method
An intermediate, aldehyde benzene technology, applied in the field of thermosetting resin and its preparation, can solve the problems of difficult and expensive polymer preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Synthesis of 3-phenyl-6-formyl-3,4-dihydro-2H-1,3-benzoxazine (AS-BOZ)
[0031] Under ice bath conditions (below 10°C), add 93.5 grams of formaldehyde solution and 30 grams of toluene to the three-necked flask, adjust the pH to 9 with sodium hydroxide solution, and add 53.4 grams of aniline dissolved in 30 grams of toluene. After stirring at low temperature for 30 minutes, 70 grams of p-hydroxybenzaldehyde was added, heated to rapidly raise the temperature of the reaction system to 80° C., and reacted at constant temperature for 5 hours. Wash with sodium hydroxide solution and water until the pH of the final washing aqueous phase is 7. The solvent toluene was removed by a vacuum rotary evaporator, and then recrystallized in toluene to finally obtain 3-phenyl-6-aldehyde-3,4-dihydro-1,3-benzoxazine as yellow needle crystals. Yield 90%; Infrared spectrum: oxazine ring (943cm -1 ), aldehyde group (1681cm -1 ); gelation time: t gel160℃ = 27 minutes and 27 secon...
Embodiment 2
[0032] Example 2 Synthesis of bis(3-phenyl-6-formyl-3,4-dihydro-2H-1,3-benzoxazine)methane (AB-BOZ)
[0033] At room temperature (lower than 20°C), add 64.8 grams of 35-40% formaldehyde solution into a three-necked bottle with a stirrer and a condenser, adjust the pH to 9 with sodium hydroxide solution, add 100 grams of toluene, ethanol 40 grams, 39.6 grams of diphenylmethanediamine, stirred for 15 minutes, then added 48.8 grams of p-hydroxybenzaldehyde, rapidly increased the temperature to 80 ° C, and stopped the reaction after 5 hours of reaction to obtain a golden yellow oil phase in the lower layer and a transparent water phase in the upper layer The phase separation system was washed with sodium hydroxide solution and water, and then the toluene was removed with a rotary evaporator to obtain a golden yellow liquid, namely bis(3-phenyl-6-aldehyde-3,4-dihydro-2H-1, 3-Benzoxazine) methane. Yield 80%, gelation time t gel160℃ = 31 minutes and 58 seconds.
Embodiment 3
[0034] Example 3 Bis(3-phenyl-6-formyl-3,4-dihydro-2H-1,3-benzoxazine)methane and bis(3-phenyl-3,4-dihydro-2H -1,3-benzoxazine) methane mixture synthesis
[0035]At room temperature (lower than 20°C), add 110 grams of 35-40% formaldehyde solution into a three-necked flask with a stirrer and a condenser, adjust the pH to 9 with sodium hydroxide solution, add 31.8 grams of phenol with stirring, and heat up to 40°C, quickly add 65 grams of diphenylmethanediamine, 105 grams of dioxane, and 35.6 grams of ethanol, the temperature of the system will automatically rise to 60°C, and continue stirring for 30 minutes. 40 grams of formaldehyde, heated up to 80 ° C, stopped the reaction after 5 hours at a constant temperature, cooled to obtain a golden yellow oil phase in the lower layer and a transparent water phase in the upper layer, washed with clear water, and then removed the solvent with a rotary evaporator to obtain a golden yellow liquid that is bis(3 -Phenyl-6-aldehyde-3,4-dihyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com