Carried nanometer bi-metal catalyst, and prepn. method and application thereof
A bimetallic catalyst and catalyst technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, chemical instrument and method, preparation of hydroxyl compounds, etc., can solve the problem of high cost, achieve low cost, high catalytic activity, and reaction short cycle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Measure 12ml (11.12g) of ethyl orthosilicate, 20ml (16g) of absolute ethanol and 6ml (5.97g) of glacial acetic acid in a 250ml flask, heat and stir, and when the solution becomes clear, add 13.5g of Ni ( NO 3 ) 2 ·6H 2 O and 6.8gCr(NO 3 ) 3 9H 2 0, stirring to obtain an alcoholic aqueous solution of nickel nitrate and chromium nitrate; the heating temperature is 80° C., kept at a constant temperature for 0.5 hours under stirring, and a blue-green viscous and uniform sol is formed, and the stirring is stopped, and the sample is packed into a cylindrical mold with one end sealed. And block the other open end with a metal mesh, then put it into an autoclave, add 280ml of absolute ethanol in the kettle, heat up with a heating rate of 5°C / min after the kettle is sealed, and the pressure in the kettle is also constantly rising until the temperature and the pressure are above the critical value of ethanol, that is, it reaches the supercritical state. When the whole system...
Embodiment 2
[0031]Measure 12ml (11.12g) of ethyl orthosilicate, 5ml (3.95g) of absolute ethanol and 6ml (5.97g) of glacial acetic acid in a 250ml flask, heat and stir, when the solution becomes clear, add 13.5g of Ni (NO 3 ) 2 ·6H 2 O and 6.8gCr(NO 3 ) 3 9H 2 O, stir to obtain the alcoholic aqueous solution of nickel nitrate and chromium nitrate; keep the constant temperature for 0.5 hours at a heating temperature of 80° C. under stirring, produce a blue-green gel, stop stirring, pour it into a beaker and cool and age for 2 hours; put the gel Put it in the tubular mold as in Example 1, block the other open end with a metal mesh, then put it into an autoclave, add 300ml absolute ethanol in the still, all the other processes are the same as in Example 1, to obtain 40% Ni, 13% Cr NiO-Cr 2 o 3 -SiO 2 Supported nano-bimetallic catalyst; its specific surface area is 450.45m by BET method 2 / g.
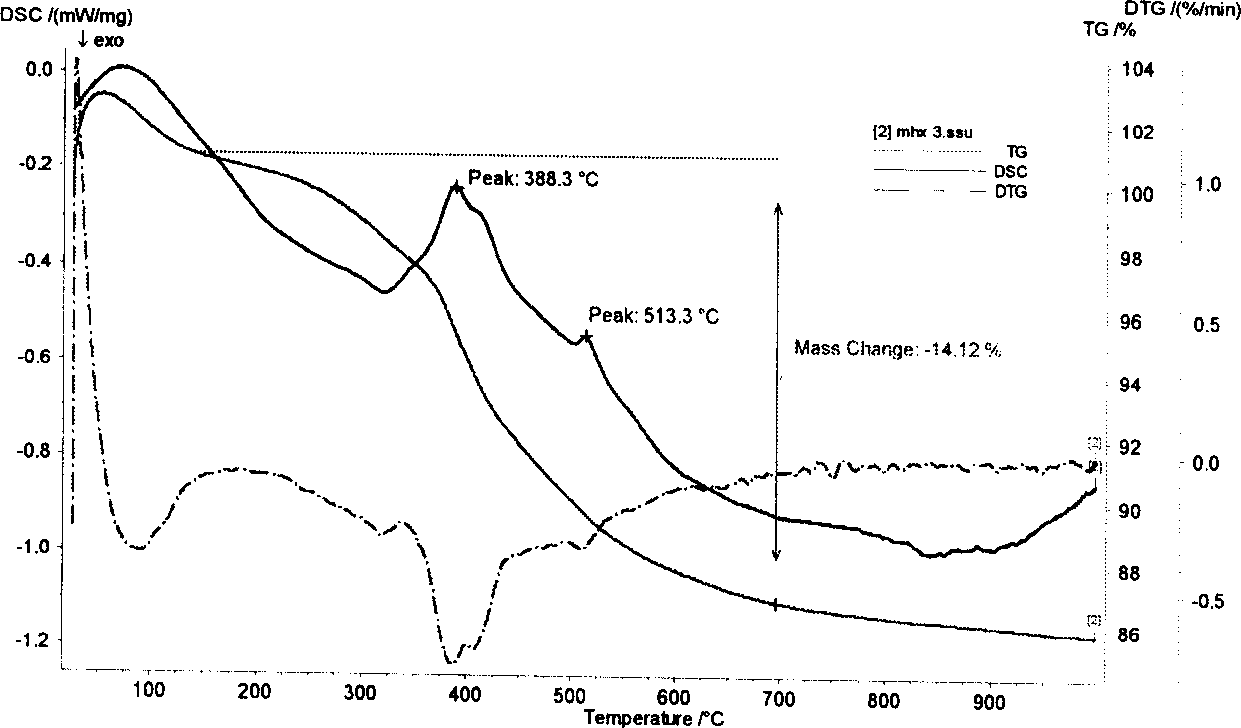
[0032] attached image 3 It is NiO-Cr obtained in embodiment 2 2 o 3 -SiO 2 TEM images...
Embodiment 3
[0038] Measure 12ml (11.12g) of ethyl orthosilicate, 40ml (32g) of absolute ethanol and 6ml (5.97g) of glacial acetic acid in a 250ml flask, heat and stir, and when the solution becomes clear, add 13.5g of Ni ( NO 3 ) 2 ·6H 2 O and 6.8gCr(NO 3 ) 3 9H 2 0, stirring to obtain an alcoholic aqueous solution of nickel nitrate and chromium nitrate; the heating temperature was 80° C. and kept at a constant temperature for 0.5 hours under stirring to form a blue-green viscous and uniform sol, stop stirring, and directly pour the sample into the sample as in Example 1. In the tubular mould, block the other open end with a metal mesh, then put it into an autoclave, add 260ml of absolute ethanol in the still, and all the other processes are the same as in Example 1 to obtain NiO containing 40%Ni and 13%Cr. -Cr 2 o 3 -SiO 2 Supported nano-bimetallic catalyst, its specific surface area is 502.96m by BET method 2 / g.
[0039] In a 100ml autoclave, add 20ml solvent methanol and 10m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com