Method for enriching, desalting protein or polypeptide in minute quantities, and carrying out analysis directly
A protein and enrichment technology, applied in the field of biochemical analysis and identification, can solve problems such as limited desalting ability, achieve the effect of high concentration efficiency, strong desalting ability, ensure reproducibility and reliability of results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
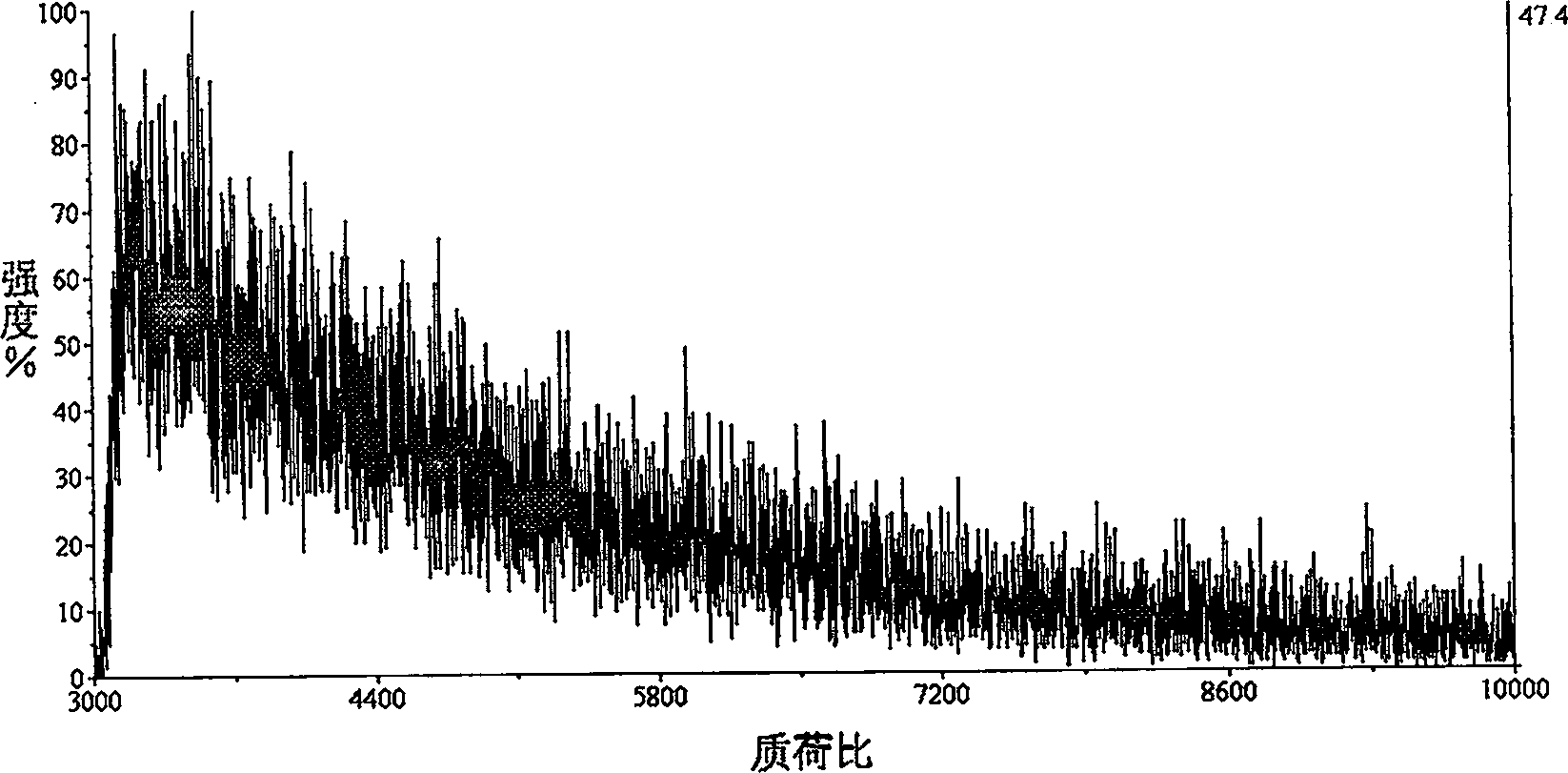
[0026] Example 1, the enrichment and MALDI TOF / MS determination of silica nanoparticles to protein solution
[0027] Take 1mL concentration 10 -8 Add 2 μL of 10 mg / mL silica nanosuspension to the ubiquitin protein sample solution of M, shake at 37°C for 30 min; suspended.
[0028] Take 0.5 μL of the aforementioned suspension and drop it on a MALDI rake plate, then add an equal volume of matrix solution, and perform mass spectrometry analysis after the crystals are dried. The mass spectrometer used is MALDI TOF / TOF (4700 Proteomics Analyzer, Applied Biosystems); the laser is Nd-YAG laser, the wavelength is 355nm, and the laser pulse frequency is 200Hz; the accelerating voltage is 20kV, and the positive ion mode linear TOF detection, the detection results are shown in image 3 .
Embodiment 2
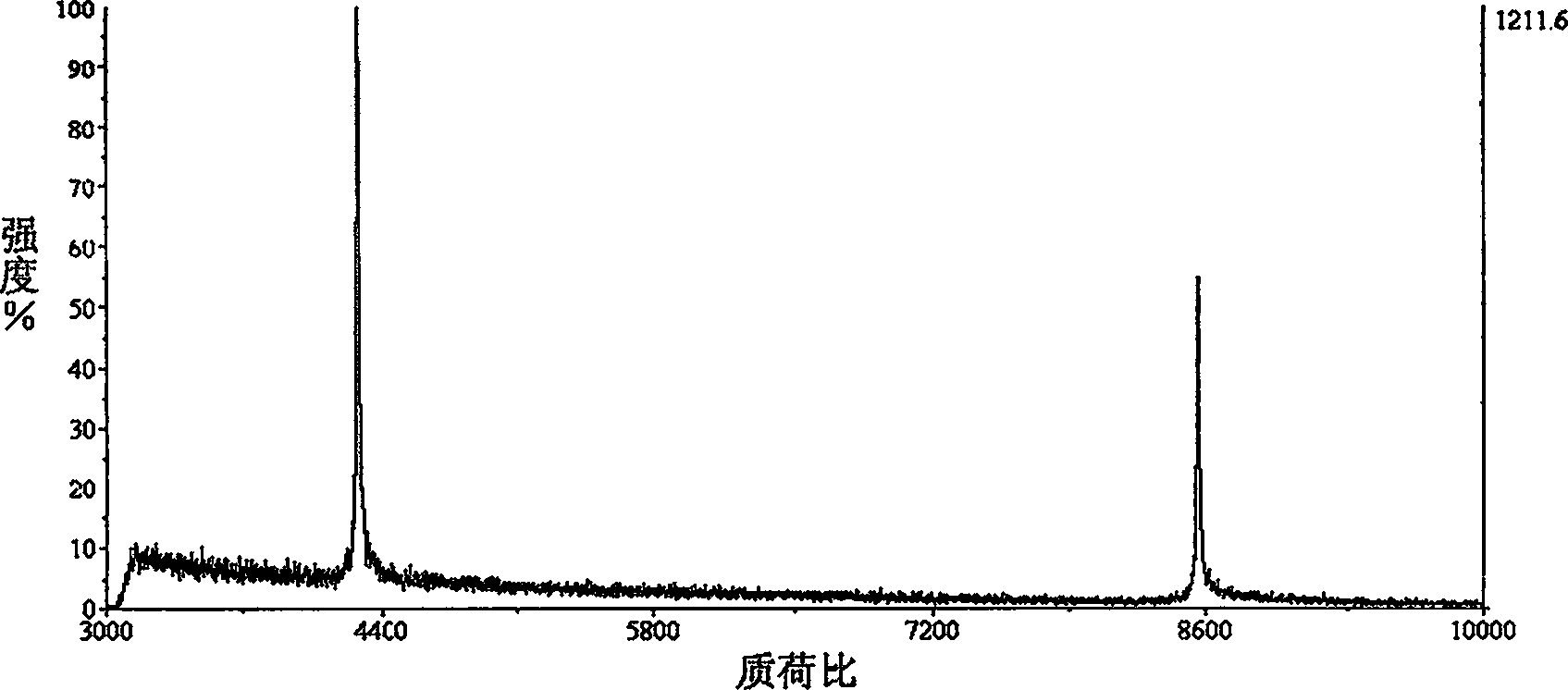
[0029] Example 2, Silica Nanoparticles Anti-salt Enrichment and MALDITOF / MS Determination of Protein Samples Containing High Concentration Inorganic Salts
[0030] Take 1 mL of Cytochrome C protein sample solution containing 4M NaCl at a concentration of Cytochrome C of 10 -7 M, add silicon oxide nano-suspension according to the method of Example 1, sample after centrifugation, positive ion mode linear TOF detection, the detection results are shown in Figure 5 .
Embodiment 3
[0031] Example 3, Silica Nanoparticles Anti-salt Enrichment and MALDITOF / MS Determination of Protein Samples Containing High Concentration Inorganic Salts
[0032] Take 1 mL of Cytochrome C protein sample solution containing 8M urea at a concentration of Cytochrome C of 10 -7 M, add silicon oxide nano-suspension according to the method of Example 1, sample after centrifugation, positive ion mode linear TOF detection, the detection results are shown in Figure 7 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com