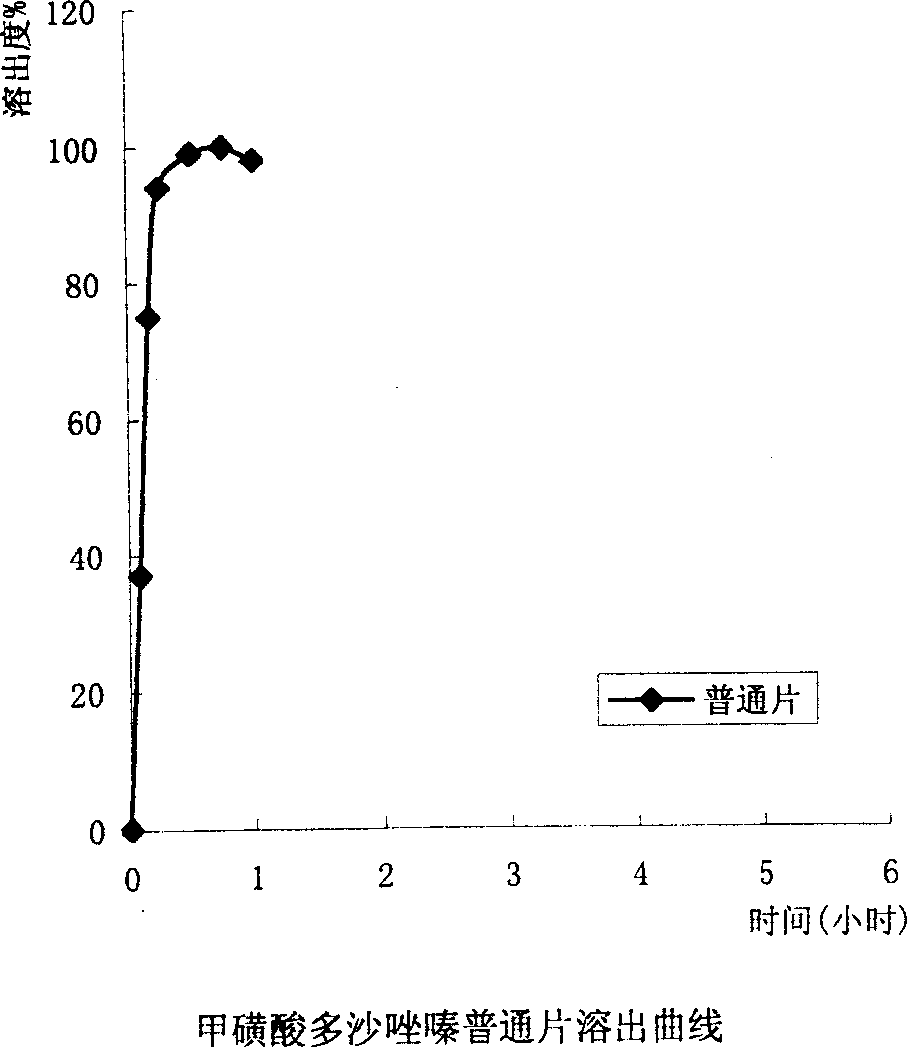
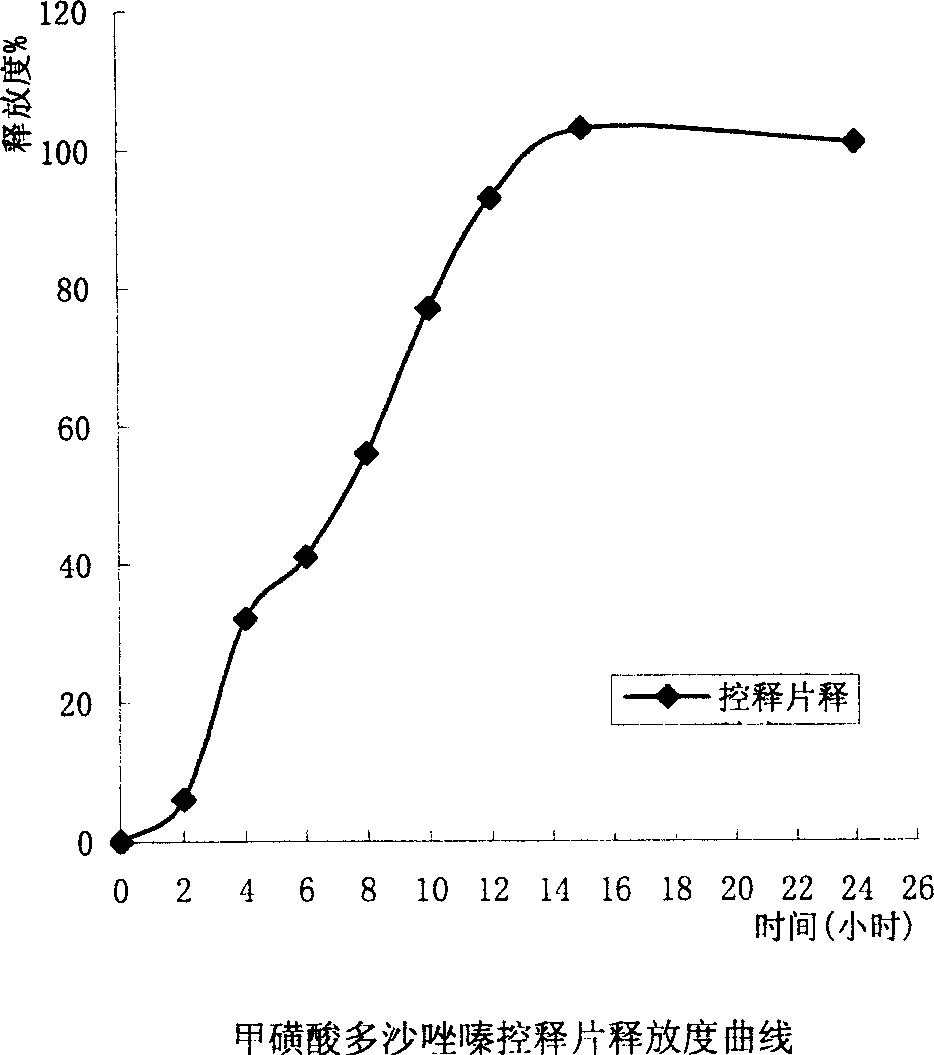
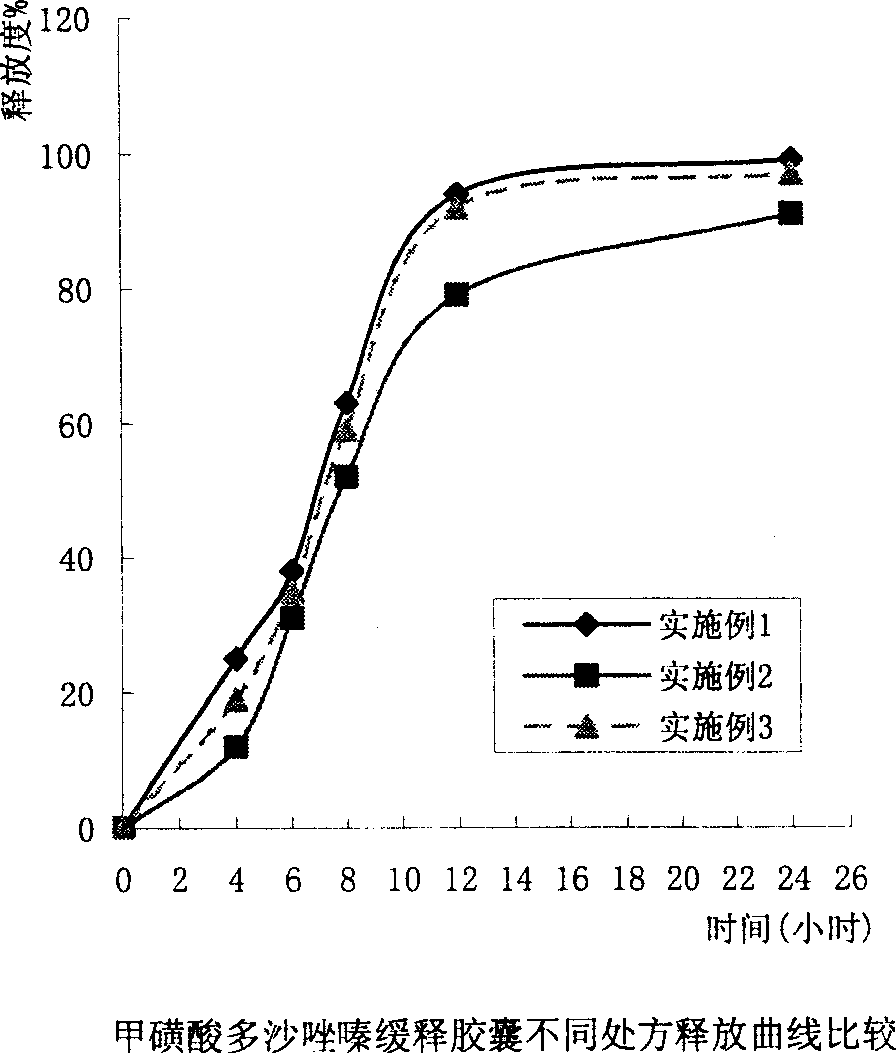
Slow released doxazosin mesilate capsule and its prepn process
A technology for doxazosin mesylate and sustained-release capsules, which is applied in the field of doxazosin mesylate sustained-release capsules, can solve the problems of cumbersome preparation process, high requirements on production conditions, complexity and the like, and achieves simple preparation process, Improve safety and efficacy, good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] prescription:
[0033] Doxazosin Mesylate 4g
[0034] Blank ball core 200g
[0035] Hypromellose 2g
[0036] Eudragit RL100 4.0g
[0037] Triethyl citrate 0.66g
[0039] Preparation:
[0040]The preparation method of the doxazosin mesylate sustained-release pellets is as follows: slowly add 2 g of hypromellose into 90 ml of 60% ethanol with a stirring device, and keep stirring until it dissolves into a clear solution. Add 4 g of doxazosin mesylate to 200 ml of anhydrous ethanol in the dark until dissolved. The above two liquids were combined as a coating liquid. Put 220g of blank pellet cores in the coating pan, set the peristaltic pump speed at 20±5 rpm, air pressure at 0.5~0.65Mpa, material bed temperature at 45~50°C, spray speed at 5ml / min, and use atomization The spray gun evenly sprays the drug-containing solution onto the ball core, and operates intermittently. After spraying the coating solution, first dry it in a coating pan for...
Embodiment 2
[0042] prescription:
[0043] Doxazosin Mesylate 4g
[0044] Blank ball core 200g
[0045] Hypromellose 2g
[0046] Eudragit RS100 4.5g
[0047] Triethyl citrate 0.66g
[0049] Preparation:
[0050] The preparation method of doxazosin mesylate sustained-release pellets is the same as in Example 1.
Embodiment 3
[0052] prescription:
[0053] Doxazosin Mesylate 4g
[0054] Blank ball core 200g
[0055] Hypromellose 2g
[0056] Eudragit RL100 2.0g
[0057] Eudragit RS100 2.0g
[0058] Triethyl citrate 0.66g
[0060] Preparation:
[0061] The preparation method of doxazosin mesylate sustained-release pellets is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com