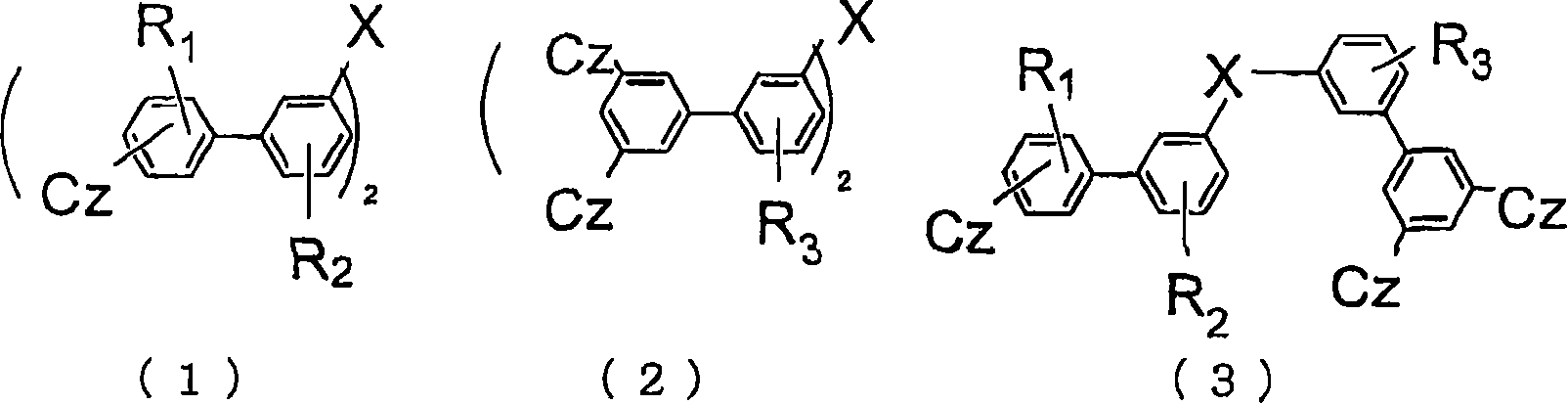
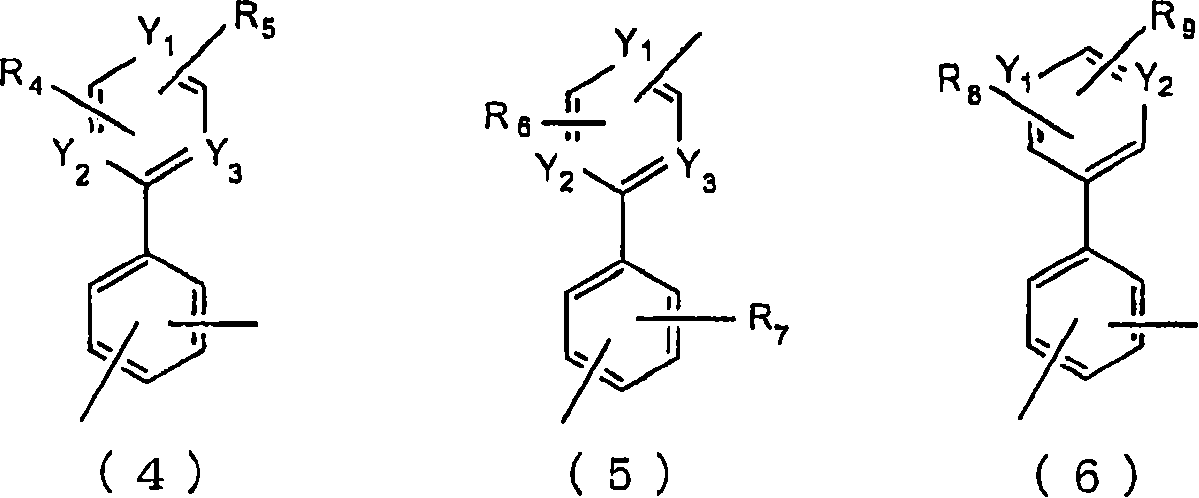
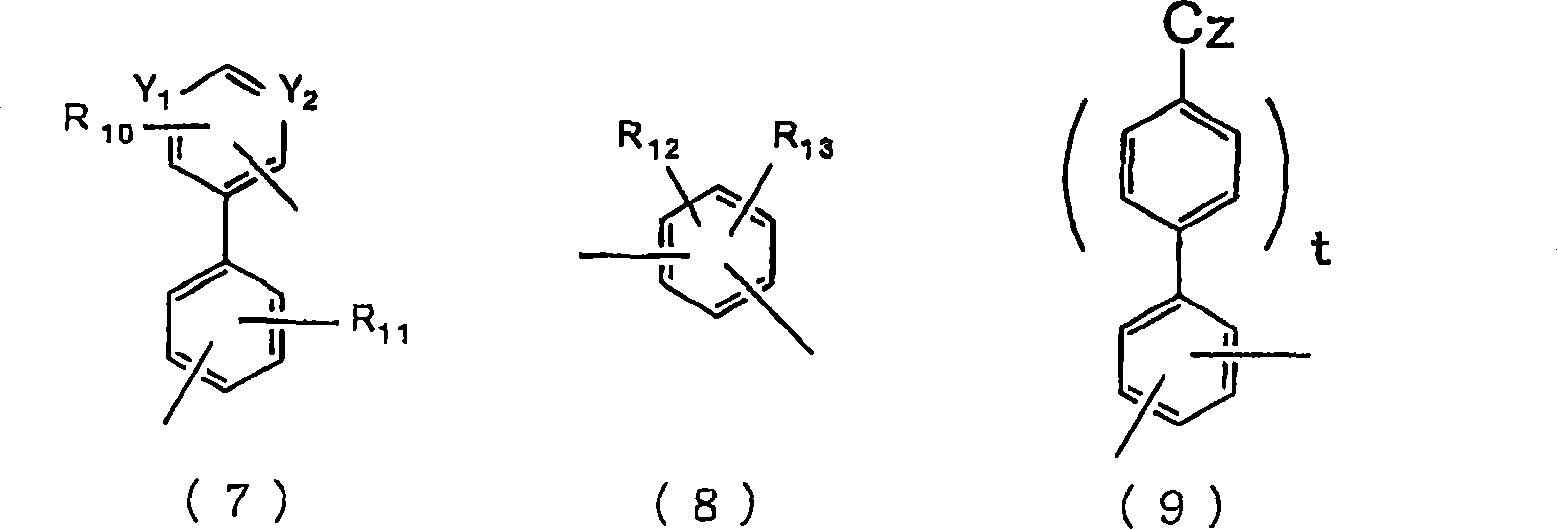
Material for organic electroluminescent device and organic electroluminescent device using same
一种电致发光元件、致发光的技术,应用在有机电致发光元件用材料,有机电致发光元件用材料及使用此材料的有机电致发光元件领域,能够解决3重态激发态能量降低、3重态激发态能量变小、发光效率降低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0182] Next, the present invention will be described in further detail using examples.
Synthetic example 1
[0183] Synthesis Example 1 (Synthesis of Compound (C5))
[0184] The synthetic route of compound (C5) is shown below.
[0185]
[0186] (1) Synthesis of intermediate (IM1)
[0187] In a 1-liter three-necked flask, add 50g (160mmol) 3,3'-dibromobiphenyl, 18.4g (110mmol) carbazole, 3.0g (16mmol) copper iodide, 46.6g (220mmol) potassium phosphate, 18.2g ( 160 mmol) of trans-1,2-cyclohexanediamine, 500 ml of 1,4-dioxane, and stirred under an argon atmosphere at 150° C. for 12 hours. Thereafter, the reaction solution was cooled to room temperature, 160 ml of water was added, and extracted three times with dichloromethane. The organic layer was dried over anhydrous magnesium sulfate, and concentrated under reduced pressure.
[0188] The residue is applied to a silica gel column, and the dicarbazole-based substances and unreacted substances are removed and purified. 11.2 g of intermediate (IM1) (28 mmol, yield 25%) were obtained. The obtained compound was measured by FD-MS (f...
Synthetic example 2
[0196] Synthesis Example 2 (Synthesis of Compound (C8))
[0197] The synthetic route of compound (C8) is shown below.
[0198]
[0199]
[0200] (1) Synthesis of intermediate (IM3)
[0201] Under an argon atmosphere, 6.2g (20mmol) of 3,5-dibromobiphenyl, 5.8g (20mmol) of p-(carbazol-9-yl)phenylboronic acid, 460mg (0.4mmol) of four Triphenylphosphine palladium 0 valent (Pd(PPh 3 ) 4 ), 100 milliliters of dimethoxyethane, 64 g (60 mmol) of a 10% by weight aqueous solution of sodium carbonate, and stirred at 78° C. for 10 hours.
[0202] After the reaction is terminated, cool to room temperature, filter the precipitated crystals, add 100 ml of toluene to the filtrate, use a separatory funnel to wash the organic layer with water and saturated saline in sequence, dry it with anhydrous magnesium sulfate, and depressurize the organic layer after filtration Concentration afforded a brown sticky solid. This was purified using a silica gel column to obtain 5.2 g of an interme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com