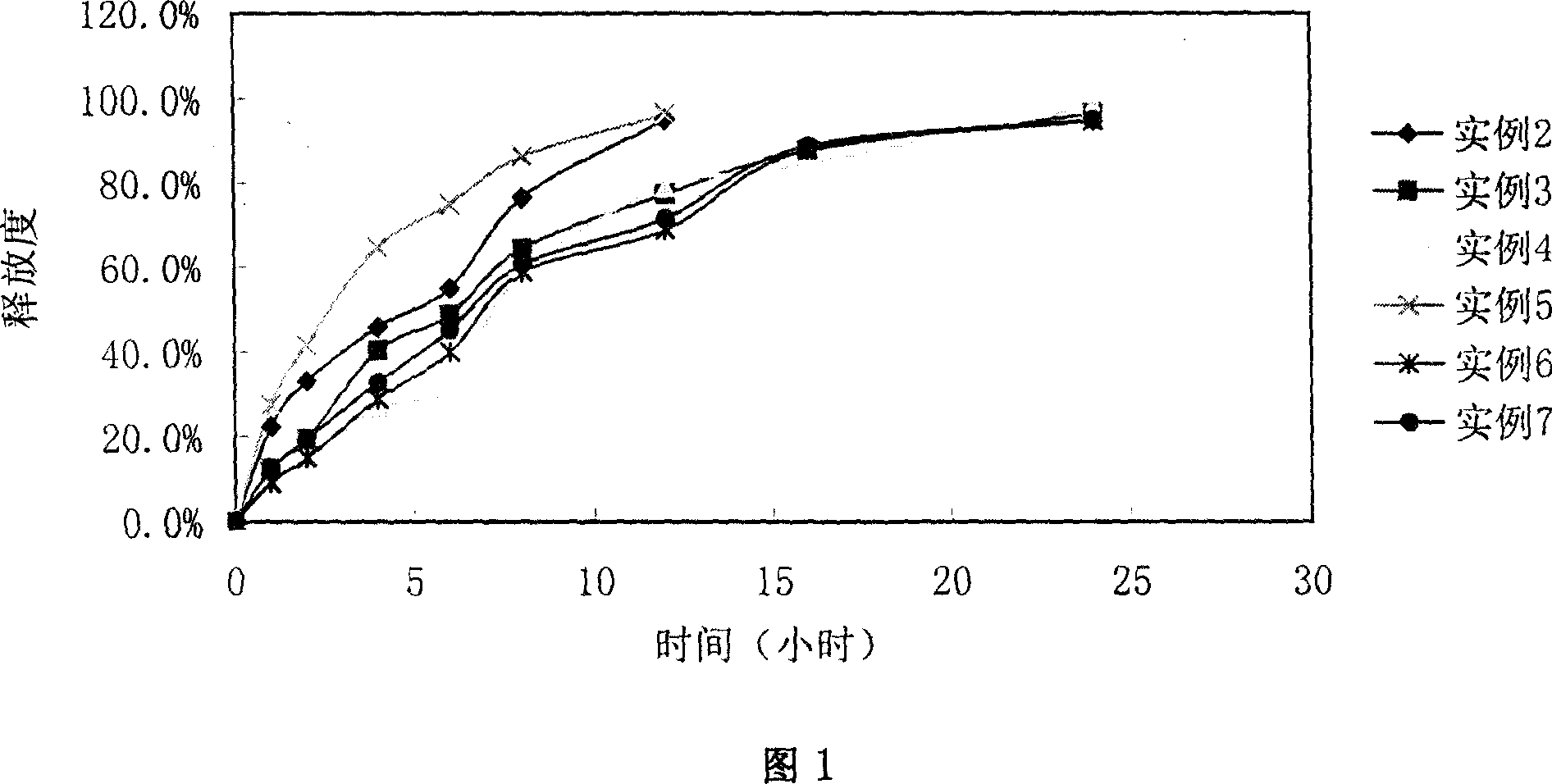
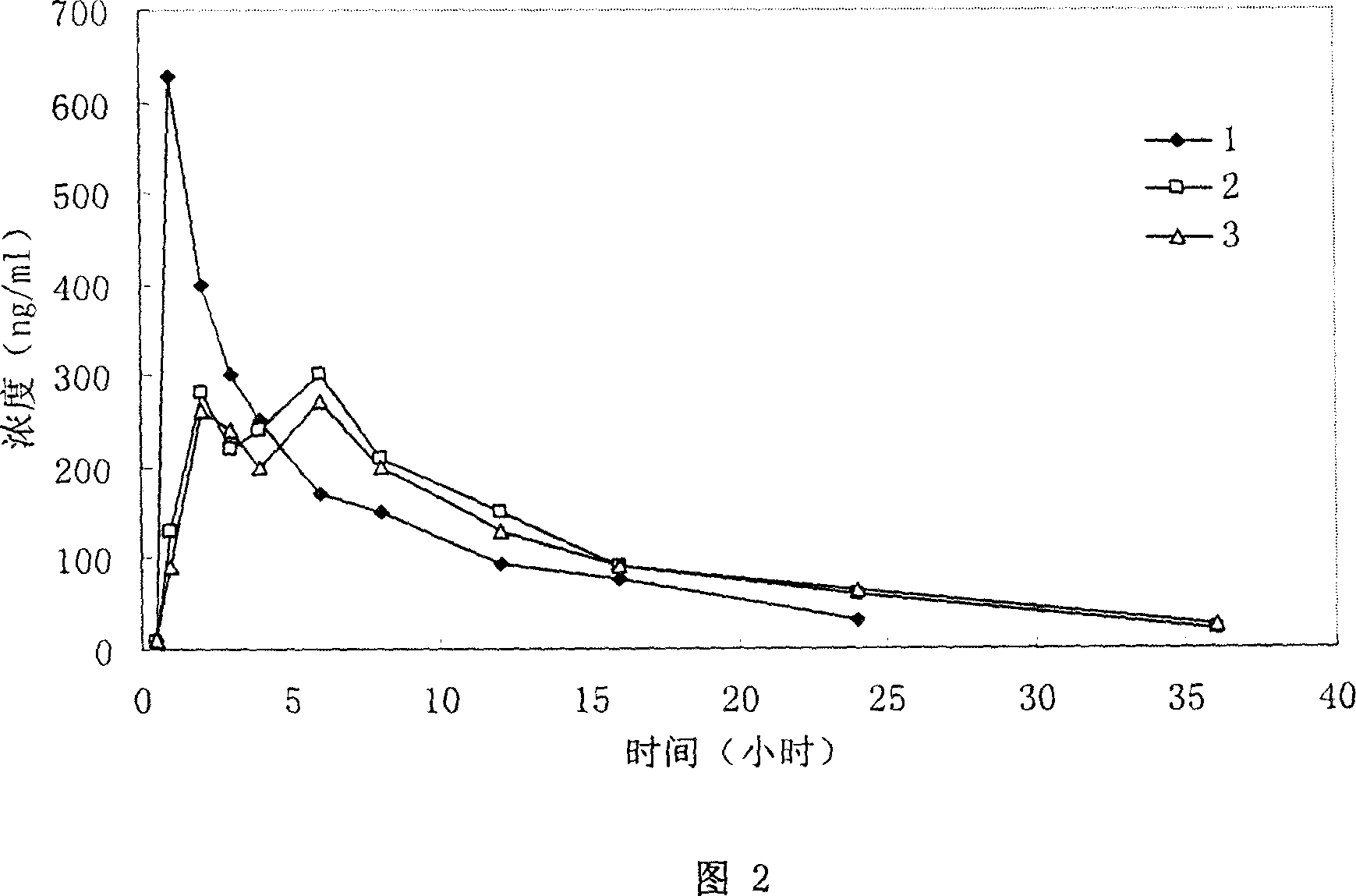
Slow release tablet comprising toraesmide active ingredient
The technology of an active ingredient, torasemide, is applied in the field of sustained-release tablets of sulfonylurea pyridine diuretics-torasemide, which can solve the problems of major side effects, achieve small side effects, low production cost, and bioavailability high degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0018] Example 1: (common tablet)
[0019] Torsemide 0.5g
[0020] Lactose 8.0g
[0021] Starch 7.0g
[0022] Polyvinylpyrrolidone K30 2.0g
[0023] water qs
[0026] Pass the main drug and auxiliary materials through a 100-mesh sieve respectively, fully mix, then weigh the prescription amount of auxiliary materials and fully mix with the main drug. Add water-based soft materials, granulate with a 20-mesh sieve, dry at 55°C, and sort through a 20-mesh sieve. Finally, add the lubricant and mix evenly for tableting.
example 2
[0030] Torsemide 0.5g
[0031] Lactose 12.0g
[0032] Acrylic resin RS100 3.0g
[0033] Stearic acid 3.0g
[0034] water qs
[0037] Pass the main drug and auxiliary materials through a 100-mesh sieve respectively, fully mix, then weigh the prescription amount of auxiliary materials and fully mix with the main drug. Then add binder to make soft material, granulate with 20-mesh sieve, dry at 55°C, and arrange with 20-mesh sieve. Finally, lubricant or hypromellose is added, mixed evenly and compressed into tablets.
example 3
[0039] The prescription is:
[0040] Torsemide 1.0g
[0041] Lactose 15.0g
[0042] Hypromellose (4000cp) 2.0g
[0043] Ethyl cellulose (100cp) 1.0g
[0044] Sodium Lauryl Sulfate 0.5g
[0045] 2% hypromellose (50% ethanol) qs
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com