Medicines of different characters and their prepn and use
A technology of traits and medicines, applied in the field of medicine, can solve the problems of calcium dibutyrylcyclic adenosine phosphate hydrate and anhydrate that have not been reported in the literature, and achieve the effect of changing functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
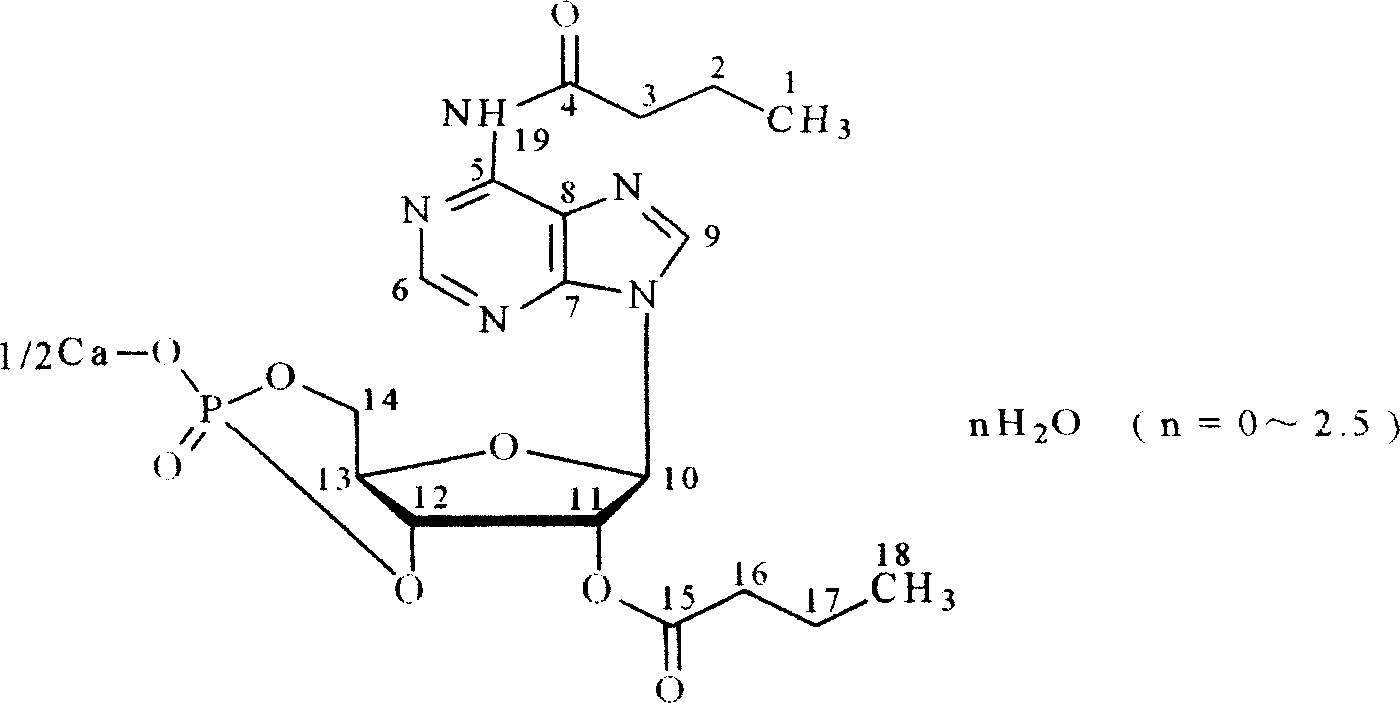
[0039] Example 1 In a dry three-necked flask, add 6.5g of cAMP triethylamine salt, 8.5ml of anhydrous pyridine, 33ml of double-distilled butyric anhydride, 200ml of chloroform, and keep stirring at 20-70°C to dissolve all the sponge, and react After the solvent is evaporated under reduced pressure, slowly add distilled water to complete the reaction at low temperature, then concentrate under reduced pressure to form a slurry, which is dbeAMP; take dibutyryl cyclic adenosine phosphate (dbeAMP) slurry and dissolve it in ethanol, add Add an appropriate amount of calcium chloride in ethanol and acetone aqueous solution, stir, mix thoroughly, dry the solvent under reduced pressure, rinse with anhydrous ether and acetone, and dry, add absolute ethanol and refined acetone to mix, add no Diethyl ether in water, placed, filtered, the solid was rinsed with anhydrous ether, drained, and dried under reduced pressure at 50-80°C for 72 hours in the presence of phosphorus pentoxide to obtain ...
Embodiment 2
[0042] Example 2 In a dry three-necked flask, add 6.5g of cAMP triethylamine salt, 8.5ml of anhydrous pyridine, 33ml of double-distilled butyric anhydride, 220ml of chloroform, and stir continuously at 28-55°C to dissolve the sponge-like substance completely, and react After the solvent is evaporated under reduced pressure, slowly add distilled water to complete the reaction at low temperature, then concentrate under reduced pressure to form a slurry, which is dbeAMP; take dibutyryl cyclic adenosine phosphate (dbeAMP) slurry and dissolve it in ethanol, add Add an appropriate amount of calcium chloride in ethanol and acetone aqueous solution, stir, mix thoroughly, dry the solvent under reduced pressure, rinse with anhydrous ether and acetone, and dry, add absolute ethanol and refined acetone to mix, add no Diethyl ether in water, set aside, filtered, the solid was rinsed with anhydrous ether, drained, and dried under reduced pressure for 30 hours to obtain off-white powder. Mel...
Embodiment 3
[0047] Example 3 In a dry three-necked flask, add 6.5g of cAMP triethylamine salt, 8.6ml of anhydrous pyridine, 35ml of double-distilled butyric anhydride, 200ml of chloroform, and keep stirring at 20-70°C to dissolve the sponge-like substance completely. After the solvent is evaporated under reduced pressure, slowly add distilled water to complete the reaction at low temperature, then concentrate under reduced pressure to form a slurry, which is dbeAMP; take dibutyryl cyclic adenosine phosphate (dbeAMP) slurry and dissolve it in ethanol, add Add an appropriate amount of calcium chloride in ethanol and acetone aqueous solution, stir, mix thoroughly, dry the solvent under reduced pressure, rinse with anhydrous ether and acetone, and dry, add absolute ethanol and refined acetone to mix, add no Diethyl ether in water, set aside, filtered, the solid was rinsed with anhydrous ether, drained, and dried under reduced pressure for 12 hours to obtain a light yellow powder. Slightly sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com