Alkaline ionic liquid and its prepn process and application
A basic ion and liquid technology, applied in the field of basic ionic liquid and its preparation, can solve the problems of poor solubility, limited use, difficult activation, etc., to achieve activity and stability promotion, good thermal stability, ultra-low vapor pressure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-12
[0036] The following examples will specifically describe the present invention, but not to further limit the present invention; all examples are operated according to the above reaction conditions and steps.
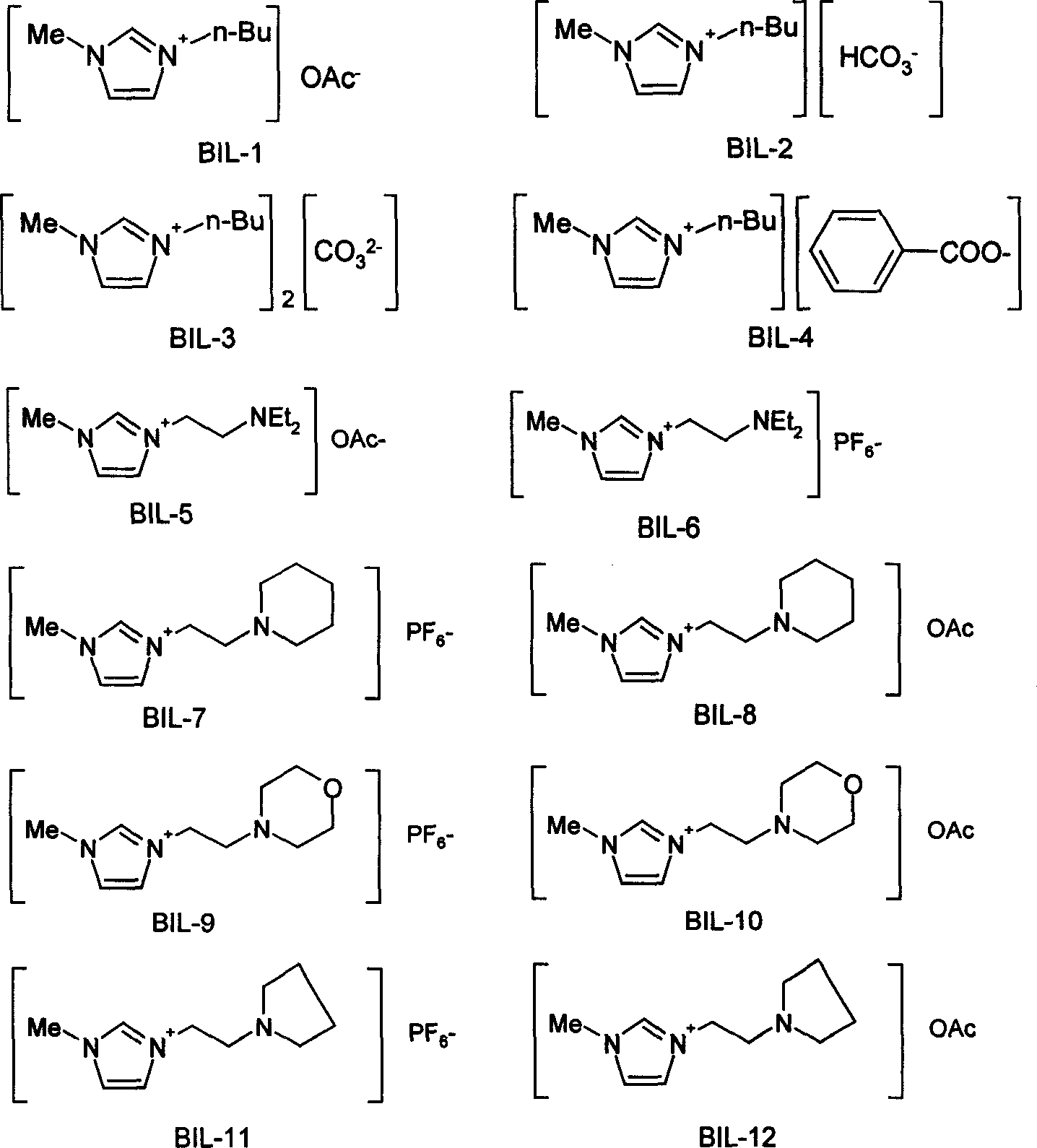
[0037] The synthesis steps of basic ionic liquid BIL1-12 are as follows. Synthesis of BIL1-4: N-methylimidazole and excess chlorobutane were refluxed in toluene for 48 hours to obtain the chloride salt of 1-butyl-3-methylimidazole. Use this chloride salt with NH 4 OAc, NH 4 HCO 3 , (NH 4 ) 2 CO 3 After ion exchange reaction with PhCOONa in acetonitrile solution, filter, remove solvent, and vacuum dry to obtain the corresponding basic ionic liquids BIL-1, 2, 3, 4, wherein BIL-4 is a white solid at room temperature. Synthesis of BIL5-12: N-methylimidazole and 2-chlorotriethylamine, 1-(2-chloroethyl)piperidine, N-(2-chloroethyl)morpholine, 1-(2-chloro Ethyl)pyrrolidine hydrochloride was refluxed in toluene for 24 hours to give the corresponding chloride salt as a sol...
Embodiment 13-27
[0040] The embodiment has investigated different acid-binding agents (traditional base NaHCO 3 、Et 3 N, the Heck coupling reaction result (table 1) of palladium-catalyzed bromobenzene and ethyl acrylate under the effect of basic ionic liquid BIL1-12)——the palladium catalyst precursor used is palladium dichloride (PdCl 2 ), the phosphine ligand is 1-butyl-3-methylimidazolium triphenylphosphine monosulfonate ([BMIM][TPPMS]), the reaction temperature is 140° C., and the reaction time is 3 hours. The results in Table 1 show that the coupling reaction is carried out in alkaline ionic liquids, the catalyst system is very stable, there is no palladium black to separate out, and the selectivity is also very good, but the conversion rate of bromobenzene is different because of the structure and composition of the ionic liquid.
[0041] Preface
No
Acid Binder
Bromobenzene conversion rate
(%)
Ethyl cinnamate selectivity
(%)
...
Embodiment 28-41
[0046] Embodiment has investigated in BIL-7 alkaline ionic liquid, four factors such as reaction temperature, palladium catalyst concentration and phosphine ligand and palladium catalyst molar ratio, and BIL-7 ionic liquid consumption etc. Heck coupling to bromobenzene and ethyl acrylate The effect of the linkage reaction.
[0047] Preface
No
temperature(℃)
Bromobenzene conversion rate
(%)
Ethyl cinnamate selectivity
(%)
1
50
5
99
2
90
31
99
3
140
43
98
4 *
200
46
98
[0048] a Bromobenzene 12mmol, ethyl acrylate 12mmol, basic ionic liquid BIL-718mmol, catalyst precursor PdCl 2 0.12mmol; PdCl 2 / [BMIM][TPPMS]=1 / 2 (molar ratio); reaction time 3 hours.
[0049] * Palladium black precipitation
[0050] Preface
No
PdCl 2 / PhBr
(mol%)
Bromobenzene conversion rate
(%)
Ethyl cinnamate s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com