Fusion protein with alpha-interferon activity and its coded gene and use
A technology of fusion protein and alpha interferon, which can be used in medical preparations containing active ingredients, peptide/protein components, hybrid peptides, etc. Mass production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1. Acquisition of a gene encoding a fusion protein with interferon-α activity
[0060] Amplify the coding gene of the fusion protein with alpha interferon activity of the present invention by PCR method, and the specific process comprises the following steps:
[0061] 1. Amplification of IFNα-2b gene
[0062] 1) Amplification of human IFNα-2b gene connected with α-factor and IgGγ1 fragment with Kex2 single protease cleavage site
[0063]The plasmid pGEM-7Z-Fa2-T7 [pGEM-7Z-Fa2-T7] containing the human IFNα-2b gene was constructed by cloning the human IFNα-2b gene (GenBank number: 184581) into the vector pGEM-7Zf(+) (Promega ) between the EcoR I and Hind III restriction endonuclease sites of the multiple cloning site to obtain a recombinant vector] as a template, in the upstream primer IFNα-2b-K-Fw (5'-CTCGAGAAAAGATGTGATCTGCCTCAAAACCCAC-3') and downstream Under the guidance of primer IFNα2b / γ1-Rv (5'-GATTTGGGCTCTTCCTTACTTCTTAAACTTTCTTG-3'), PCR amplified human I...
Embodiment 2
[0131] Example 2, Expression of fusion protein gene with alpha interferon activity in Pichia pastoris and purification of expression product
[0132] 1. Construction of a Pichia pastoris expression vector having a fusion protein gene with alpha interferon activity
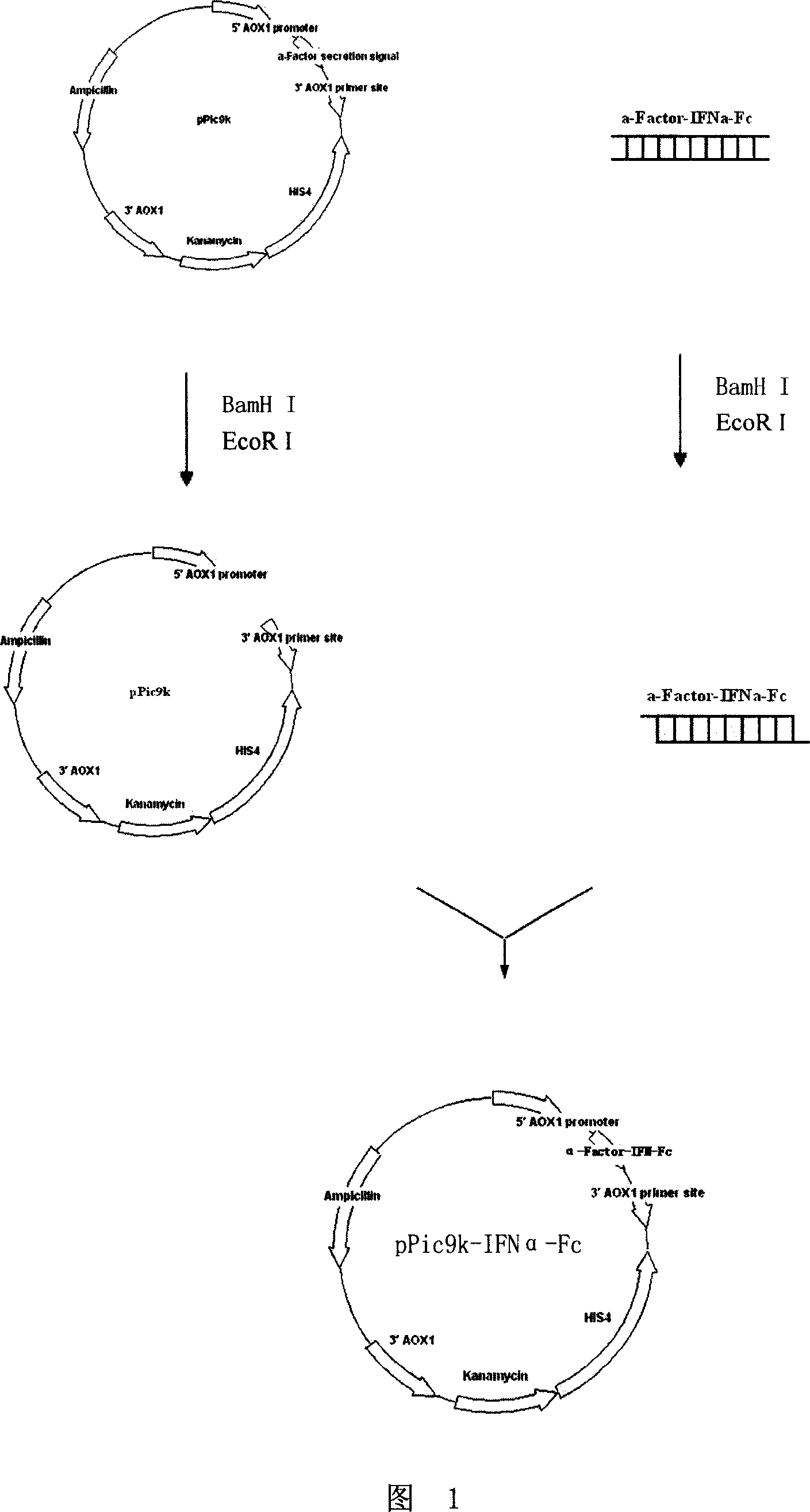
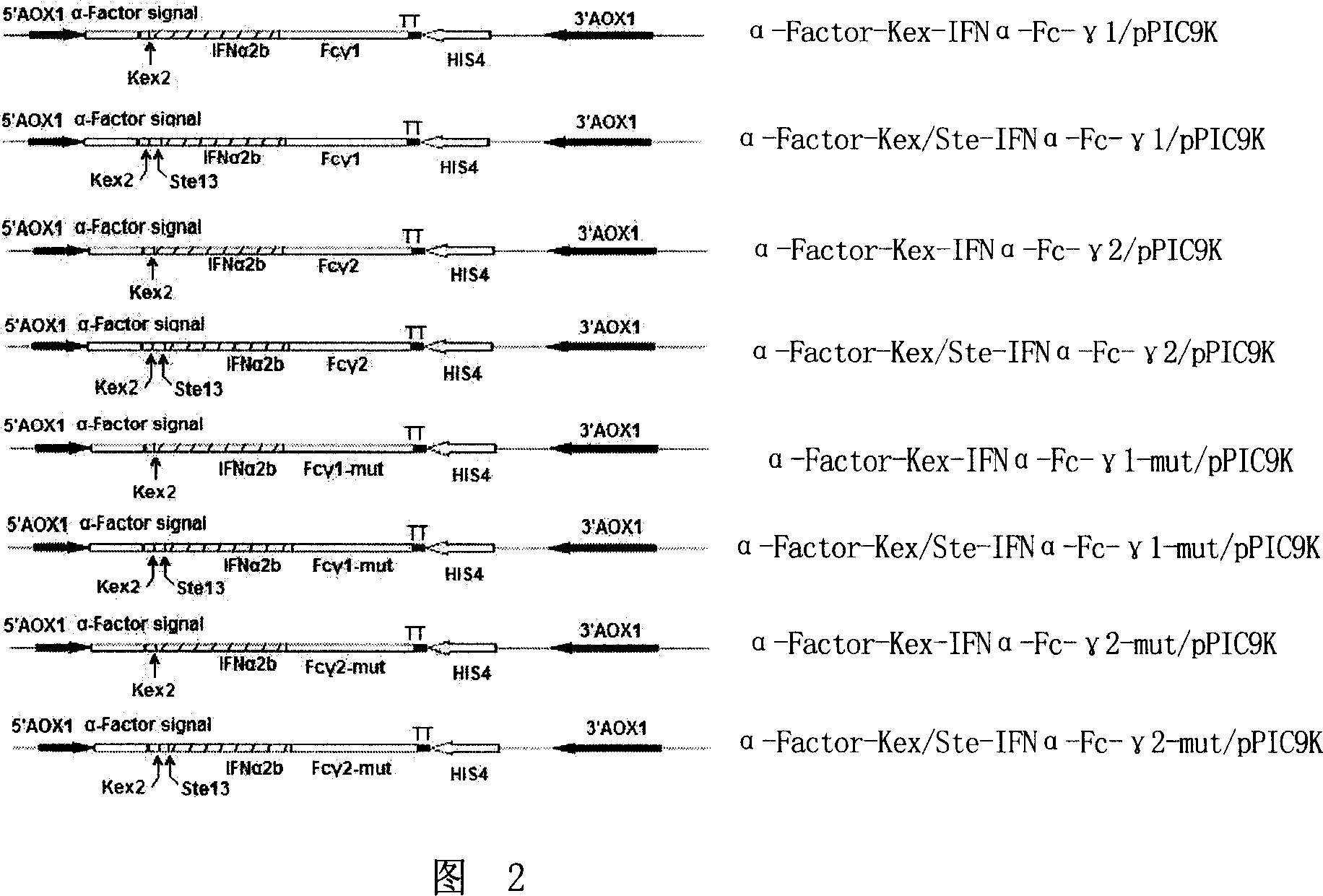
[0133] Referring to Fig. 1 constructing the Pichia pastoris expression vector of the fusion protein gene with alpha interferon activity, specific method is: use restriction endonuclease BamH I and EcoR I to the 8 gene fragments that embodiment 1 step seven obtains (collectively referred to as alpha -Factor-IFNα-Fc) were double-enzymatically digested, and then the 8 digested fragments were respectively connected with the plasmid pPic9k that had been double-digested with the same enzyme with T4 DNA ligase, and the ligated products were transformed into E. coli DH5α competent cells, and screened Positive recombinants, plasmids were extracted, sequenced, and the sequencing results were consistent with the expected resu...
Embodiment 3
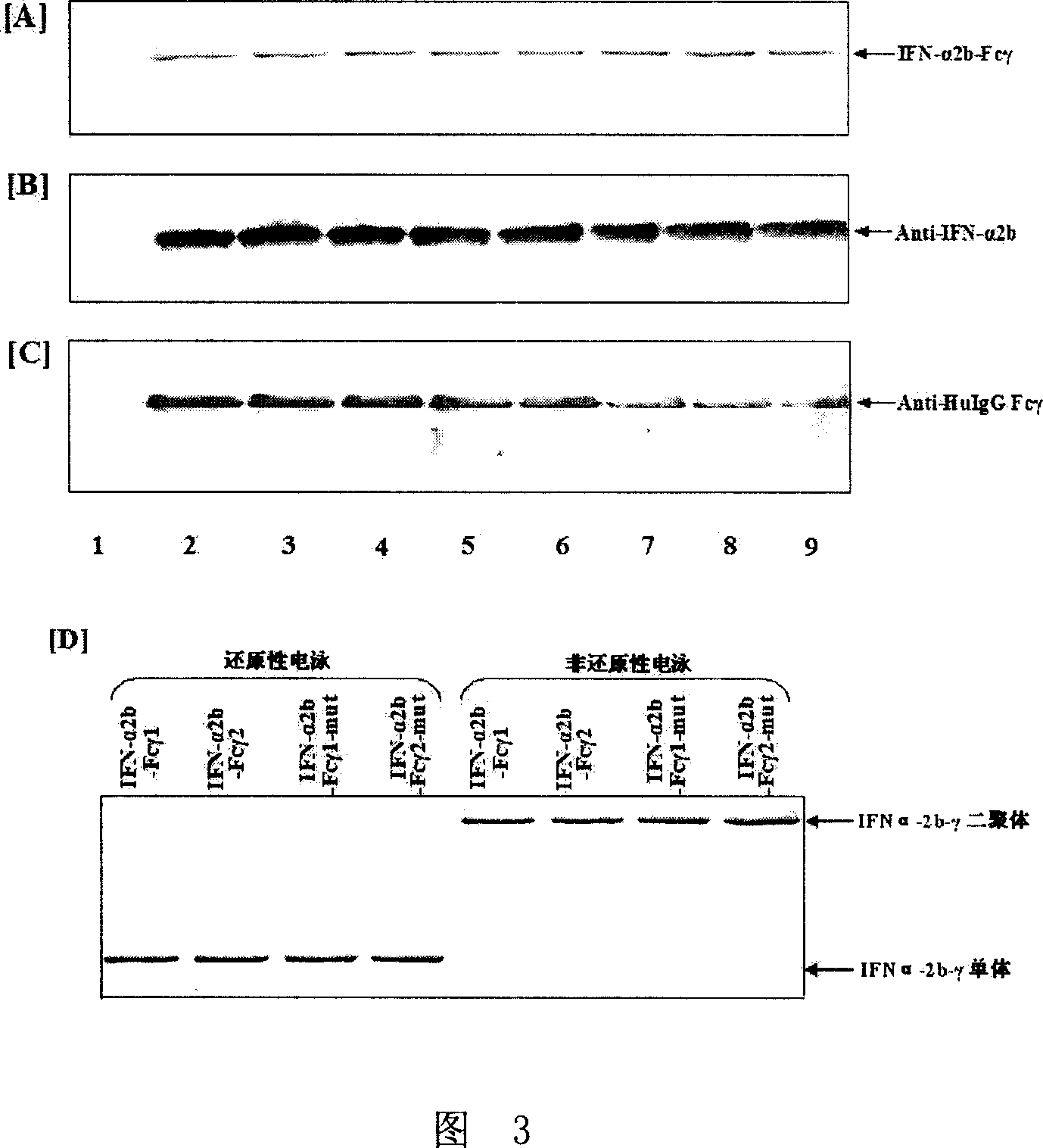
[0165] Example 3, Detection of IFN-α activity of the fusion protein of the present invention
[0166] The fusion protein obtained through expression in Example 2 was tested for IFN-α activity using the human amniotic cell Wish strain (Wish cell) / vesicular stomatitis virus (VSV virus) system. 10% calf serum DMEM medium (purchased from Invitrogen Company) was made into a suspension with a cell concentration of 3.5 × 105 / mL, added to a 96-well cell culture plate, 100ul / well, in 5% CO 2 After culturing for 4-6h in a 37° C. incubator, add 100 ul of the fusion protein sample obtained through expression in Example 2 through 4-fold gradient dilution into each well (the sample diluent is DMEM culture fluid containing 7% calf serum), and at the same time Set up IFNα-2b standard substance control and blank control wells, and incubate at 37°C for 18-24h. After the cultivation, the supernatant was discarded, and the VSV virus (100TCID50) diluted in DMEM medium containing 3% calf serum was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com