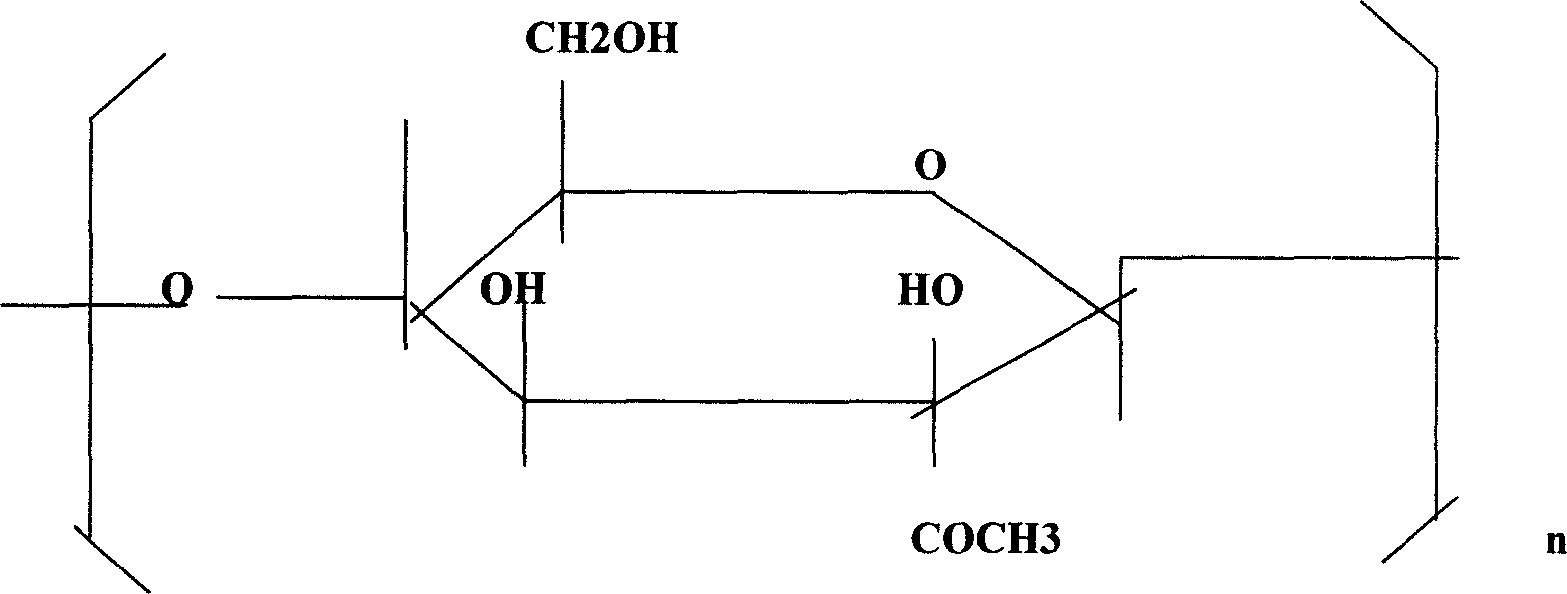
Application of yew amylose in pharmacy
A technology of yew polysaccharides and drugs, which is applied in the direction of antineoplastic drugs, drug combinations, and pharmaceutical formulas, and can solve problems such as limited biological resources, poor water solubility, and toxic and side effects, so as to expand the application team, enhance tolerance, and avoid resources. wasteful effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0132] Take 500g of dry branches and leaves of yew plant, crush them to below 40 mesh, sieve, soak in ethyl acetate solution overnight, dry in the air, pass through 0.9Nacl solution, 85% ethanol solution, 95 degree hot water, 1% ammonium oxalate solution, 5% NaoH solution, 20% NaoH solution, sequentially extracted to obtain crude polysaccharide extract, freeze-dried, deproteinized, then freeze-dried, purified by ultrafiltration chromatography to obtain primary pure polysaccharide, then vacuum-dried, purified by molecular chromatography to obtain high-purity polysaccharide 0.7037 g, dissolved in 61 g of distilled water, added with necessary carriers approved by the field of pharmaceuticals, routinely sterilized, and made into a yew polysaccharide oral liquid with a concentration of 1%.
preparation example 2
[0134] Take 500g of the dry branches and leaves of the yew plant, crush them to below 40 mesh, sieve, soak in ethyl acetate solution overnight, dry in the air, pass through 0.9Nacl solution, 85% ethanol solution-95 degree hot water, 1% ammonium oxalate solution, 5% NaoH solution, 20% NaoH solution, sequentially extracted to obtain crude polysaccharide extract, freeze-dried, deproteinized, then freeze-dried, purified by ultrafiltration chromatography to obtain primary pure polysaccharide, then vacuum-dried, purified by molecular chromatography to obtain high-purity polysaccharide 0.6853 g, dissolved in 11 g of distilled water, added with necessary carriers approved by the pharmaceutical field, routinely sterilized, and made into a 5% yew polysaccharide oral liquid.
preparation example 3
[0136] Take 500g of the dry branches and leaves of the yew plant, crush them to below 40 mesh, sieve, soak in ethyl acetate solution overnight, dry in the air, pass through 0.9Nacl solution, 85% ethanol solution-95 degree hot water, 1% ammonium oxalate solution, 5% NaoH solution, 20% NaoH solution, sequentially extracted to obtain crude polysaccharide extract, freeze-dried, deproteinized, then freeze-dried, purified by ultrafiltration chromatography to obtain primary pure polysaccharide, then vacuum-dried, purified by molecular chromatography to obtain high-purity polysaccharide 0.6626g, and then sub-packed into hard capsules each containing 1mg of necessary carrier starch approved by the field of medicine, 0.05mg of magnesium stearate, and 0.45mg of the active ingredient of yew polysaccharide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com