Fuel cell, electronic equipment, movable body, power generation system and cogeneration system
A fuel cell and fuel technology, applied in the direction of fuel cells, biochemical fuel cells, solid electrolyte fuel cells, etc., can solve the problems of resource consumption, high temperature heating, difficult practical application, etc., to simplify the fuel supply system, realize resource recycling society, easy to handle effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
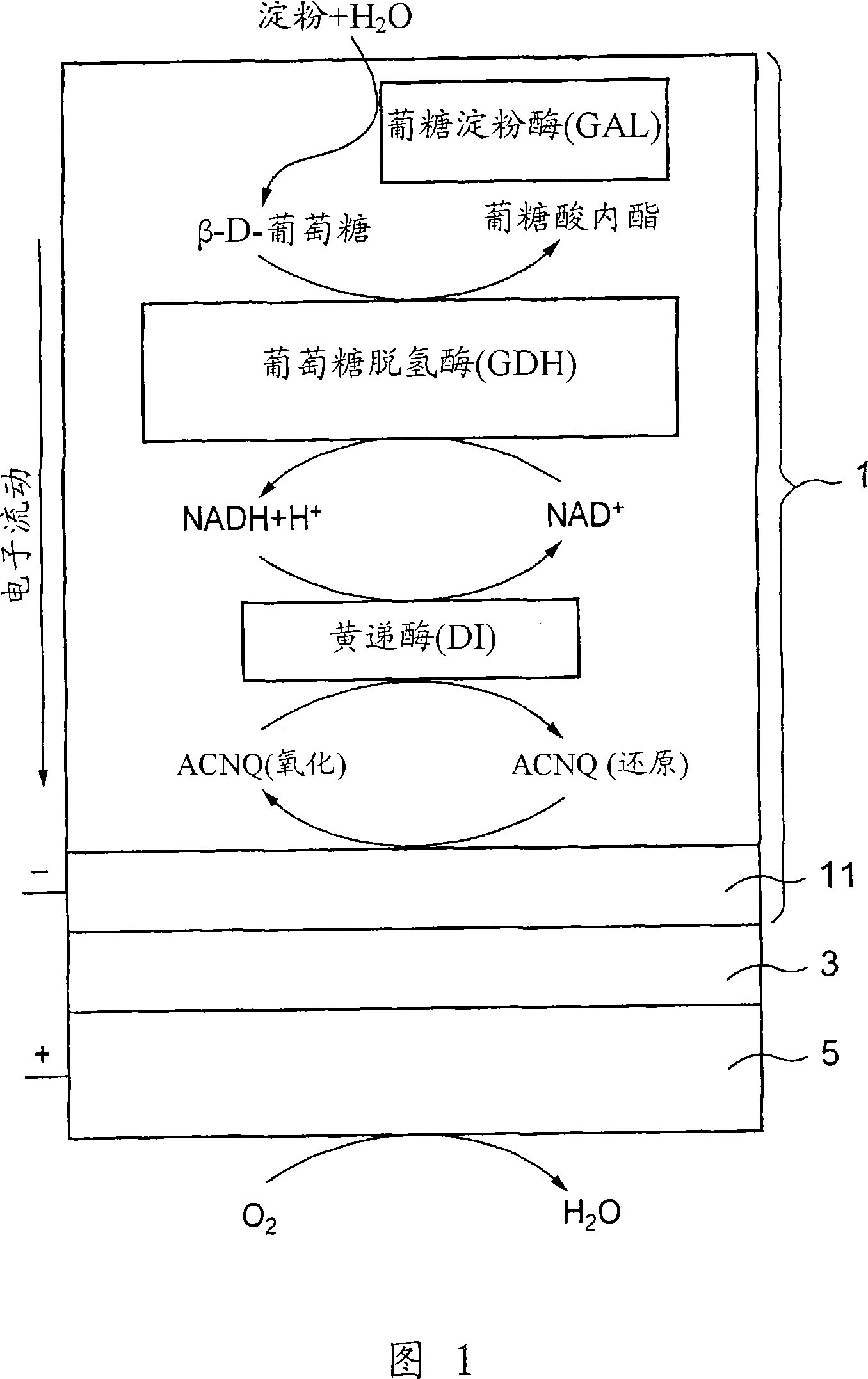
[0127] 3 μl of diaphorase (DI) (UNITIKA LTD., from Bacillus stearothermophilus) in phosphate buffered saline (83 μM), 6 μl of glucose were applied dropwise on a glassy carbon (GC) electrode (BAS, φ=3.0 mm). Dehydrogenase (GDH) (TOYOBO LTD.) in phosphate buffered saline (60 μM), 3 μl of glucoamylase (GAL) (Oriental Yeast Co., Ltd.) in phosphate buffered saline (1.4 mM), 3 μl of poly- L-lysine (PLL) in water (1%), 2 μl of NADH in phosphate buffered saline (0.4 M), 2 μl of ACNQ in ethanol (28 mM) and 3 μl of glutaraldehyde (GA) in water (0.125%), and They were mixed with each other and air-dried at room temperature, and then washed with distilled water to prepare a GAL / GDH / NADH / DI / ACNQ immobilized electrode (see FIG. 1 ).
[0128] The immobilized electrode prepared in this way is used as a working electrode, the Ag / AgCl electrode is used as a reference electrode, the Pt electrode is used as a pole plate, and the electrolytic cell made of polytetrafluoroethylene with a volume of 1...
Embodiment 2
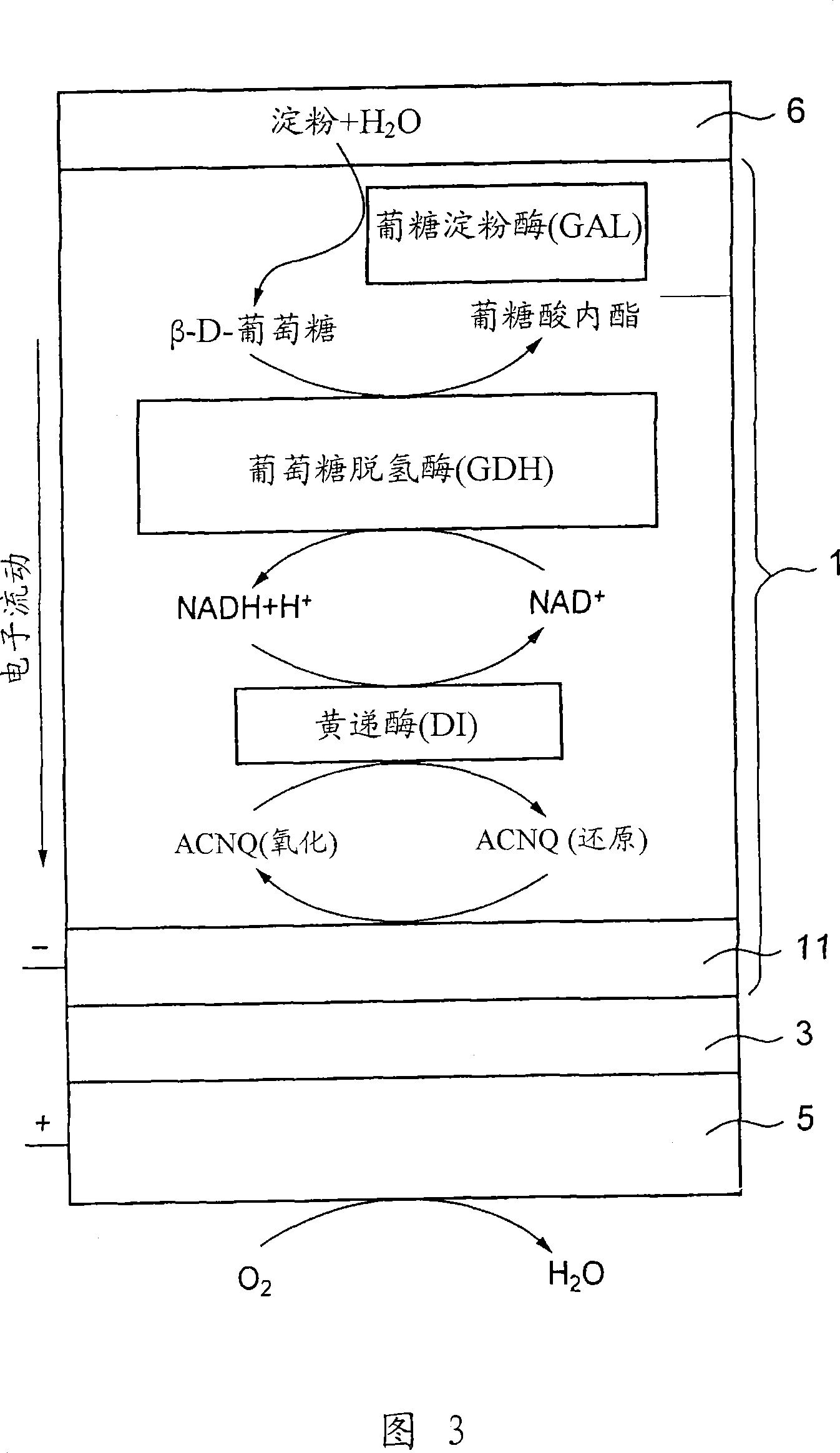
[0130] Electrochemical measurements were performed in substantially the same manner as in Example 1, except that 5 mg of the substance obtained by gelatinizing a 50% starch phosphate buffer solution at 70° C. was coated on the GAL / GDH / NADH / DI prepared in Example 1 / ACNQ was immobilized on the electrode, and the reaction solution was changed to 1 ml of 0.1 M phosphate buffer solution (pH 7; I.S.=0.3).
Embodiment 3
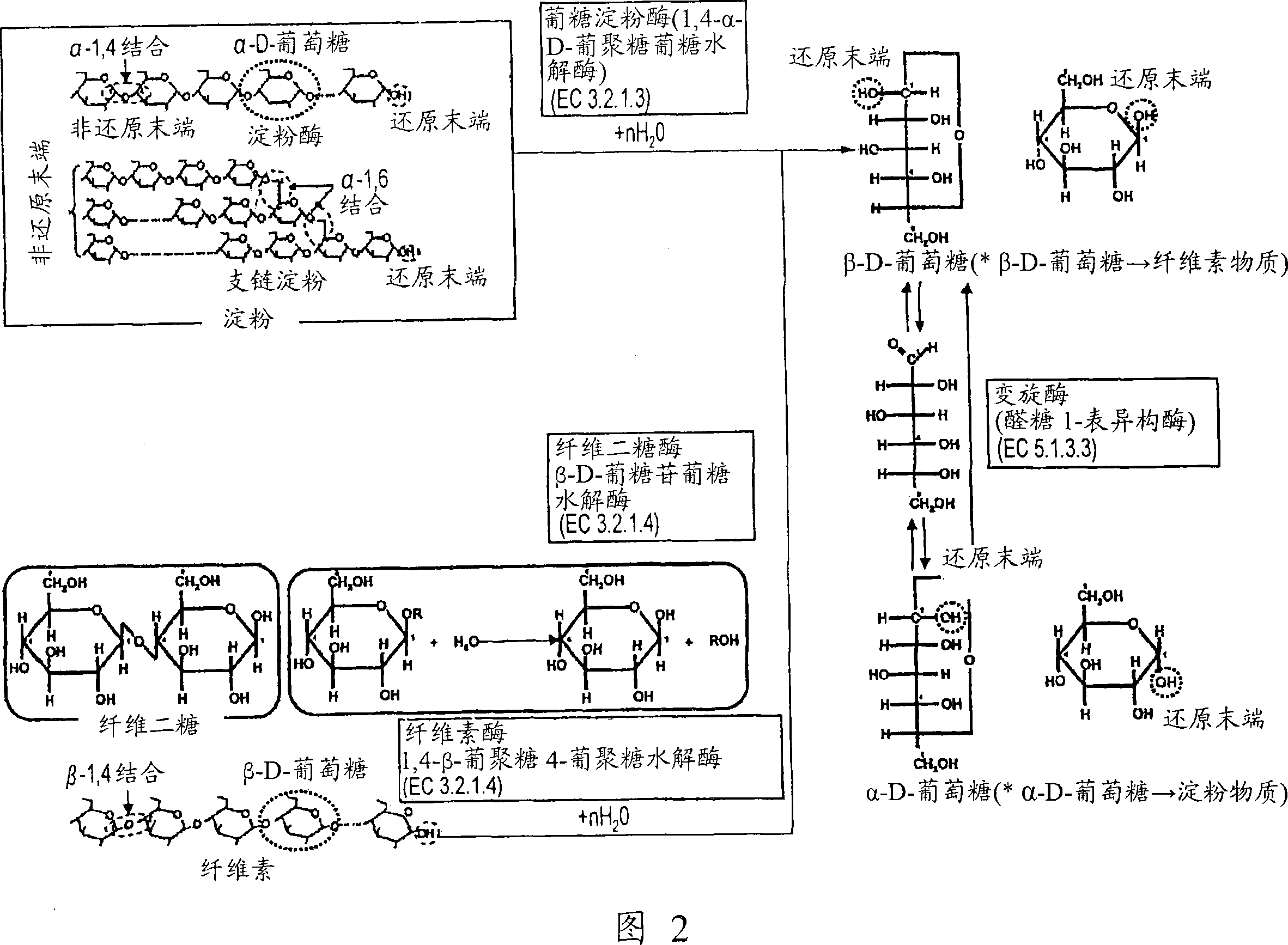
[0140] For the fuel electrode 1 having starch gel 6 immobilized thereon as fuel and glucoamylase (GAL) as decomposing starch into glucose, CV measurement was performed under the same conditions as used in Example 1. The results are shown in Figure 12 (curve a). In FIG. 12 , as a reference, the CV measurement results (curve b) in the case where the glucose solution is used as fuel are also shown. As can be seen from FIG. 12 , compared with the maximum current obtained when glucose solution (glucose concentration: 200 mM) was used as fuel, when starch glue 6 was used as fuel, a considerable current could be obtained. As mentioned above, this result indicates a very high concentration of glucose on the electrode 1 surface. In addition, the reason why the current increases with the lapse of time is that starch is gradually hydrolyzed by glucoamylase (GAL), and thus the concentration of glucose on the electrode surface increases with the lapse of time. Curve b has a shape only fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current density | aaaaa | aaaaa |
| Power density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com