Adeno-associated vector compositions for expression of factor VIII
a technology of adenoassociated vectors and compositions, applied in the direction of vectors, drug compositions, viruses, etc., can solve the problems of infection and septicemia, intracranial and/or extracranial hemorrhage, and very dangerous bleeding situations, so as to improve or restore the blood clotting time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Dual Vector Plasmid Construction
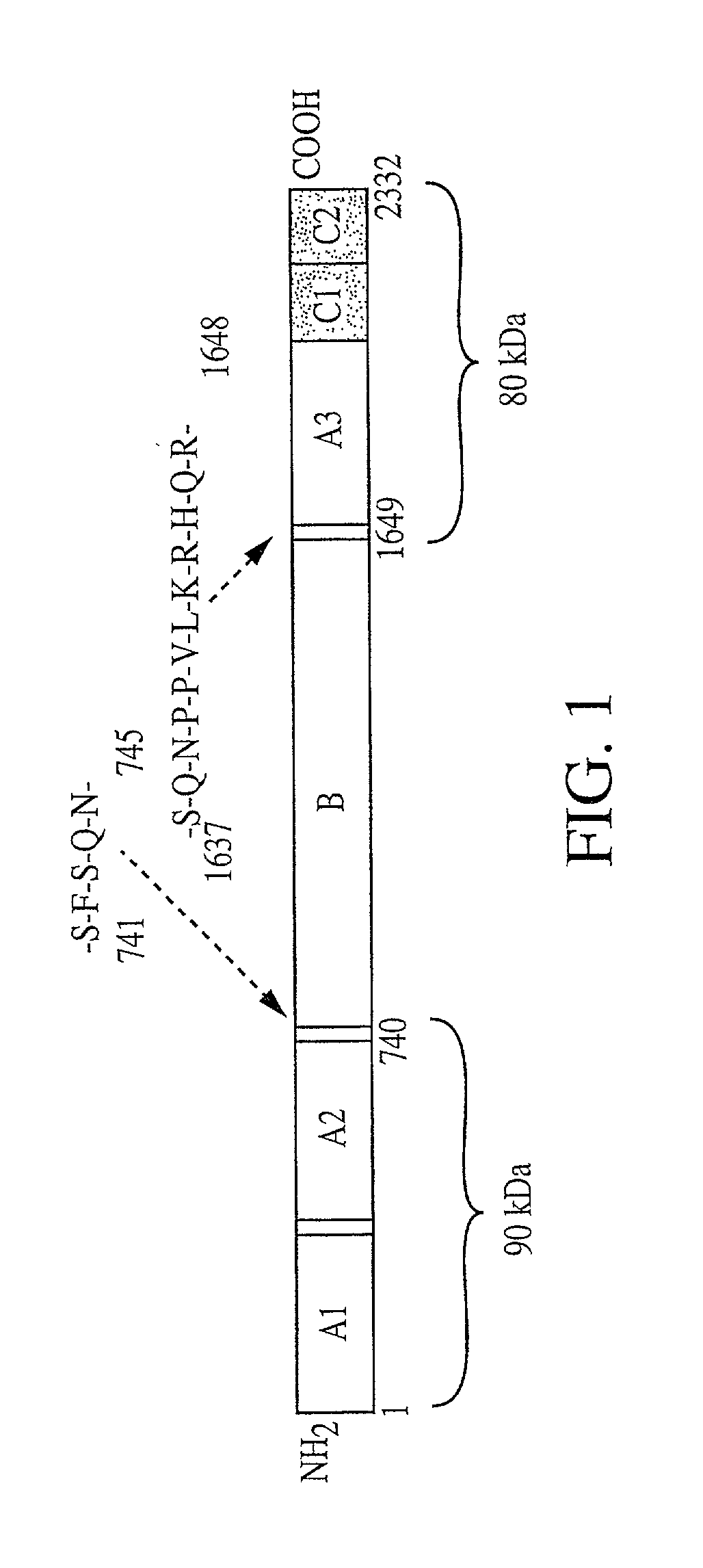
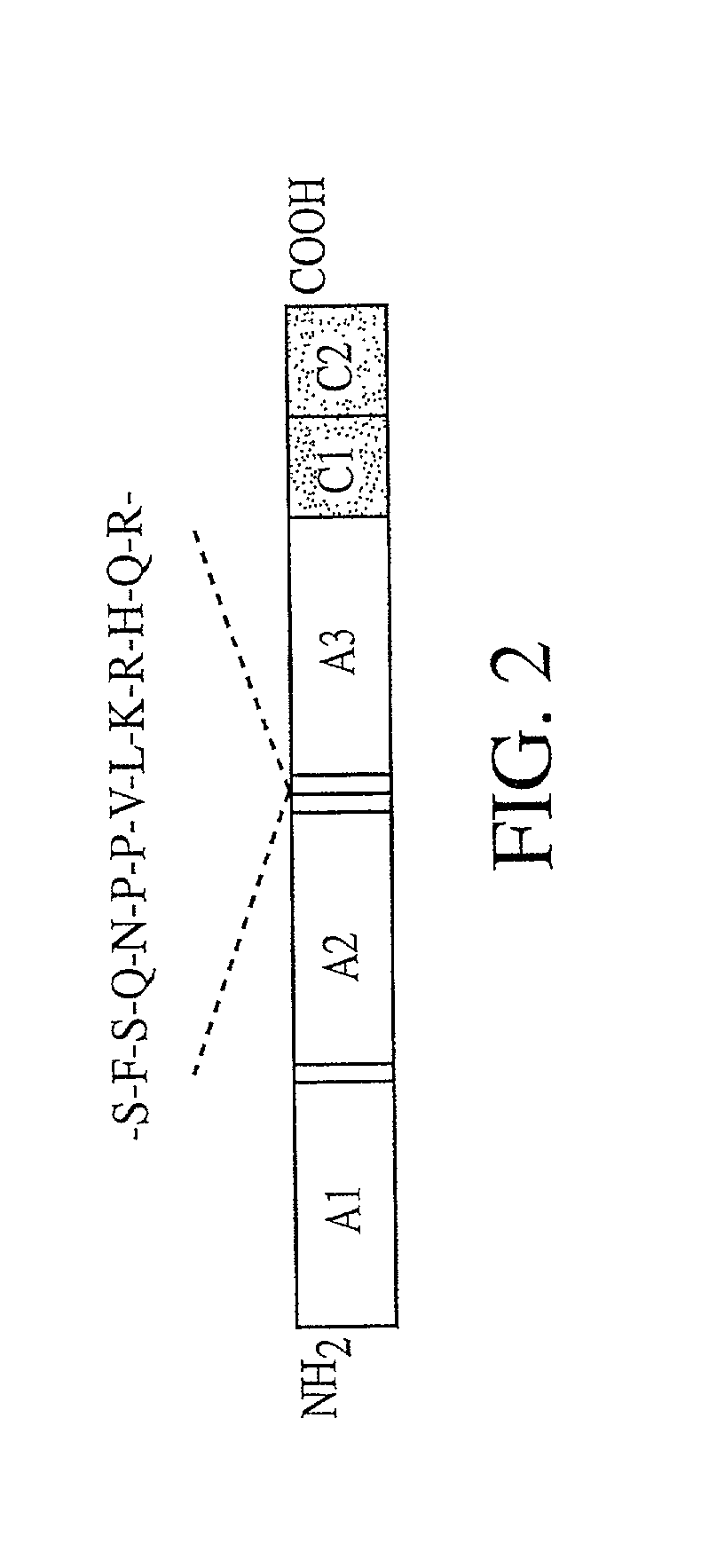
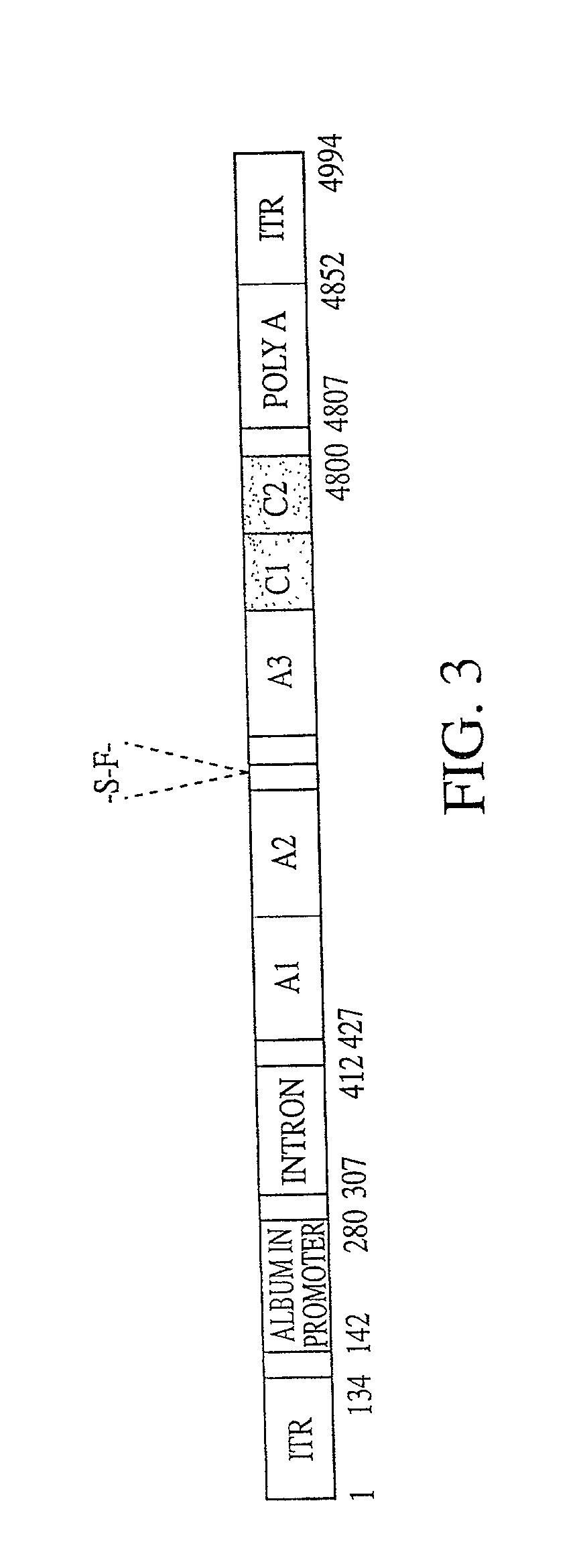
[0129] The heavy and light chains of human Factor VIII (hFVIII) were assembled according to those reported by Yonemura et al (Yonemura et al., Prot. Engineer., 6:669-674 [1993]) and cloned as expression cassettes into AAV vectors. Both vectors contain the promoter and the first non-coding intron (from -573 to +985) from the human elongation factor 1.alpha. (EF1.alpha.) gene (Uetsuki et al, J. Biol. Chem., 264:5791-5798 [1989]; and Kim et al., Gene 9:217-223 [1990]). Each vector also contains the first 57 base pairs of the FVIII heavy chain encoding the 19 amino acid signal sequence. The heavy chain construct encodes the Al and A2 domains and 5 amino acids from the N terminus of the B domain. The light chain vector encodes 85 amino acids of the carboxy terminal B domain, in addition to the A3, C1, and C2 domains. Both vectors utilize the human growth hormone (hGH) polyadenylation signal. The expression cassettes were inserted between AAV ITRs. The init...
example 2
Single Vector Plasmid Construction
[0132] The plasmid pAAV-F8-1 construct containing both the light and heavy chains of factor VIII was constructed as follows. A PCR fragment, Z8, containing cloning sites, 5'-splicing donor site of a synthetic intron based on EF1.alpha. and immunoglobulin G (IgG) intron sequences, Kozak sequence and the first 16 nucleotides of the human blood coagulation factor VIII (FVIII) coding sequence was generated using oligonucleotides Z8S and Z8A. The sequences of the nucleic acids is shown below:
[0133] Oligonucleotide Z8S:
[0134] 5' cccaagcttgcggccgcccgggtgccgcccctaggcaggtaagtgccgtgtgtggttcc 3' (SEQ ID NO:1)
[0135] Oligonucleotide Z8A:
[0136] 5' ccgctcgagcagagctctatttgcatggtggaatcgatgccgcgggaaccacacacggc 3' (SEQ ID NO:2)
[0137] PCR fragment Z8:
[0138] 5' cccaagcttgcggccgcccgggtgccgcccctaggcaggtaagtgccgtgtgtggttcccgc ggcatcgattccaccatgcaaatagagctctgctcgagcgg 3' (SEQ ID NO:3)
[0139] Nucleic acid Z8 was inserted into pZERO-2 (Invitrogen) between HindIII and Xhol site...
example 3
Virion Production
[0163] AAV vectors were produced from these plasmids using the Ad free system as previously described in U.S. Pat. No. 5,858,351; U.S. Pat. No. 5,846,528; U.S. Pat. No. 5,622,856; and Matsushita et al., Gene Ther 5:938 (1998) all of which are hereby incorporated by reference. Briefly, 293 cells (ATCC, catalog number CRL-1573) were seeded in 10 cm dishes at a density of 3.times.10.sup.6 cells per dish in 10 ml medium and incubated at 37.degree. C. with CO.sub.2 and humidity. After an overnight incubation, cells were approximately seventy to eighty percent confluent.
[0164] The cells were then transfected with DNA by the calcium phosphate method, as is well known in the art. Briefly, 7 .mu.g of AAV vector containing the Factor VIII coding region, 7 .mu.g of pladeno5 which supplies the accessory functions, and 7 .mu.g of 1909 AAV helper were added to a 3 ml sterile, polystyrene snap cap tube using sterile pipette tips. Then, 1.0 ml of 300 mM CaCl.sub.2 (JRH grade) was a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com