Method of and apparatus for manufacturing the metallic iron
a technology of metallic iron and manufacturing method, which is applied in the direction of manufacturing converters, furnace types, furnaces, etc., can solve the problems of reducing the purity of metallic iron (iron quality), increasing the amount of slag, and lowering the yield of refined molten iron
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
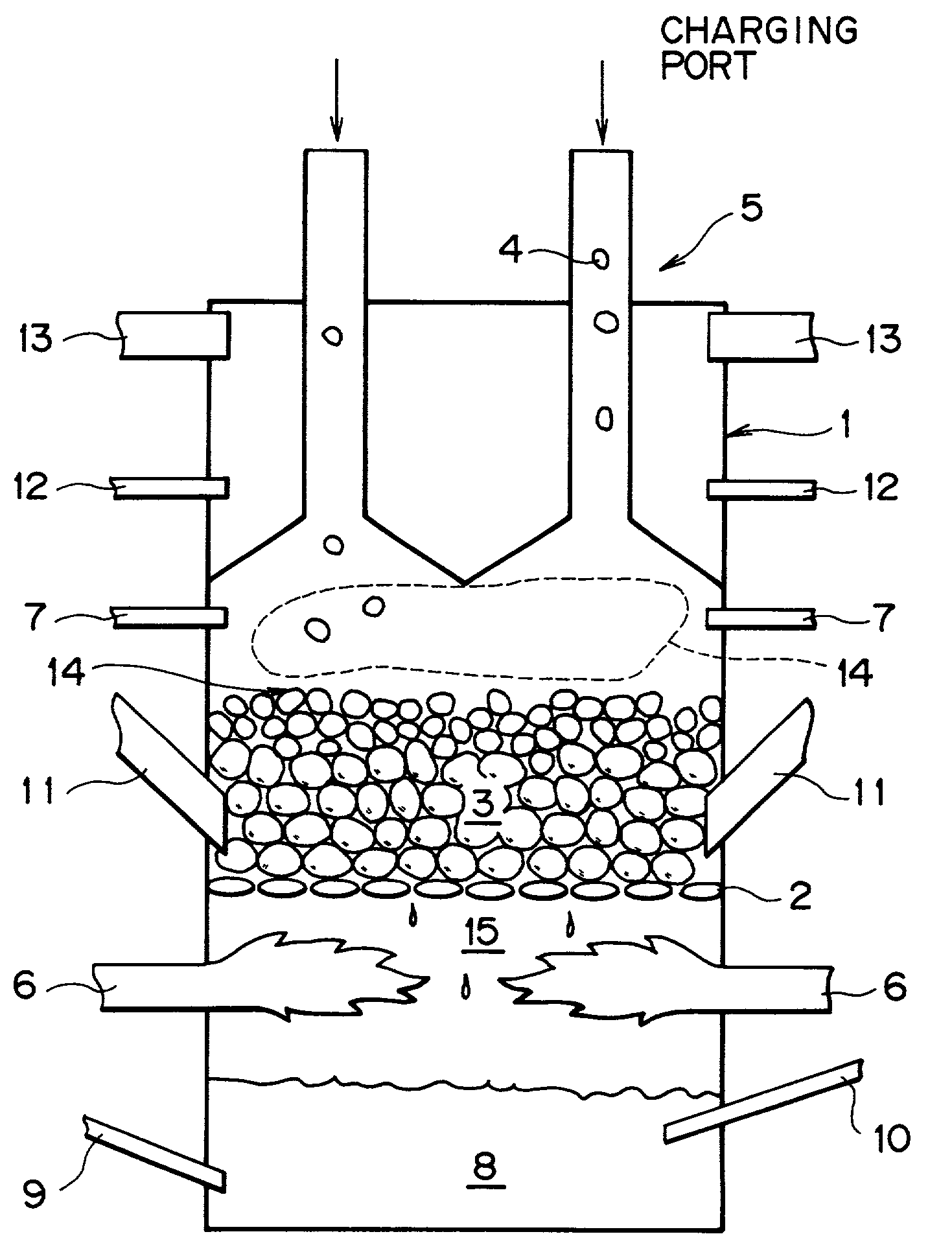
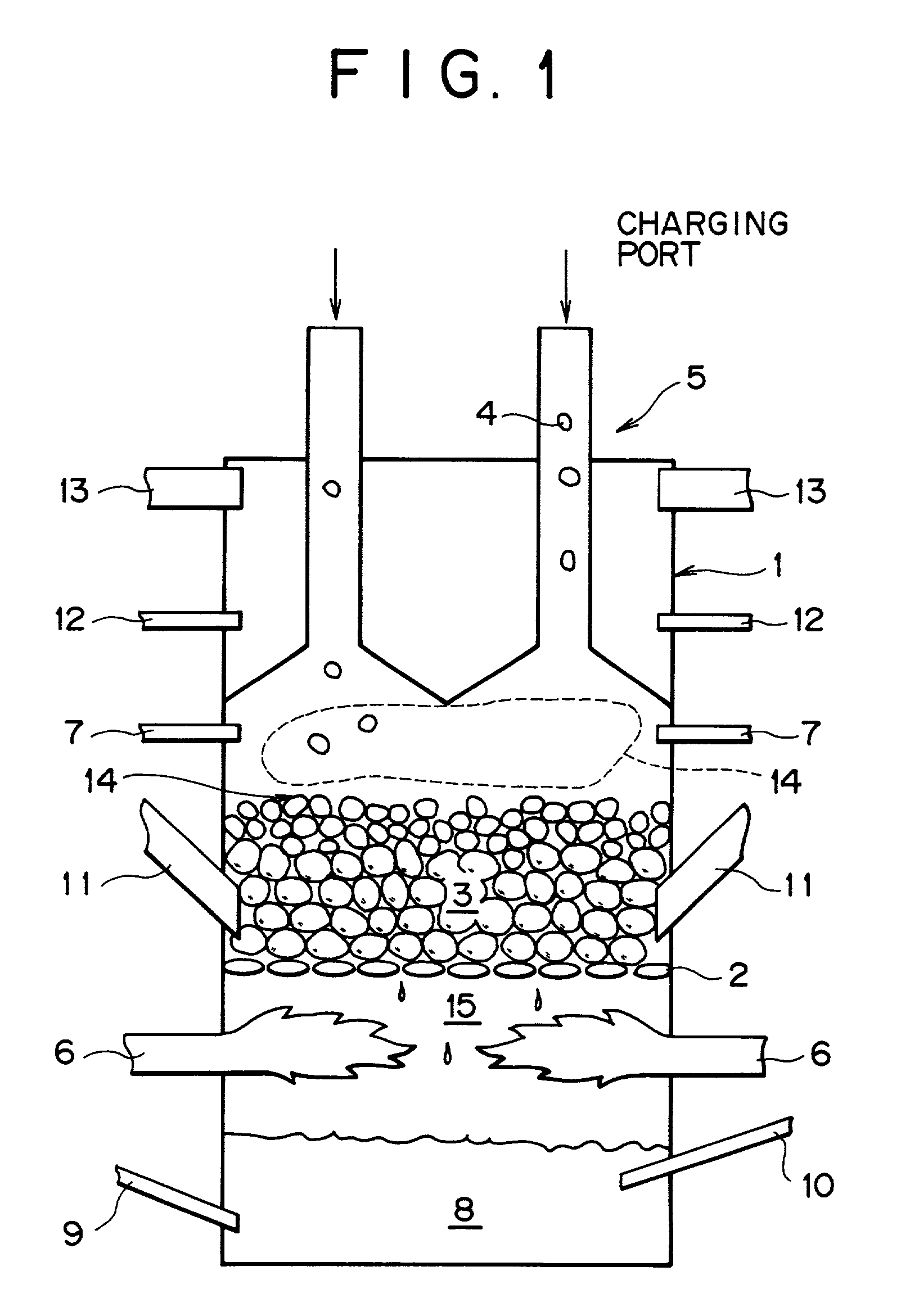
[0067] The following experiment was conducted by using a packed bed shown in FIG. 1. A water cooled fire grate (2) as a water-cooled rostor is disposed in a furnace body (1) of 60 cm inner diameter.times.2 m height, refractories comprising alumina (average particle size: 15 mm) were charged on the fire grate through a refractory charging mechanism (11) and, subsequently, the refractories are appropriately supplemented to keep the thickness of the refractory layer to 20 cm and heated by the heat of combustion formed in the lower portion of the furnace. Iron ore powder, coal powder and binder (each of 75 .mu.m or less in average grain size) of the following chemical composition, which were uniformly mixed at the following ratio and then formed into pellets (spheres), were supplied on the refractories through a compact charging mechanism (5) (charged height: 5-40 cm). A fuel (natural gas) mixed with air was introduced in a fuel combustion space below the fire grate (2) through a fuel s...
example 2
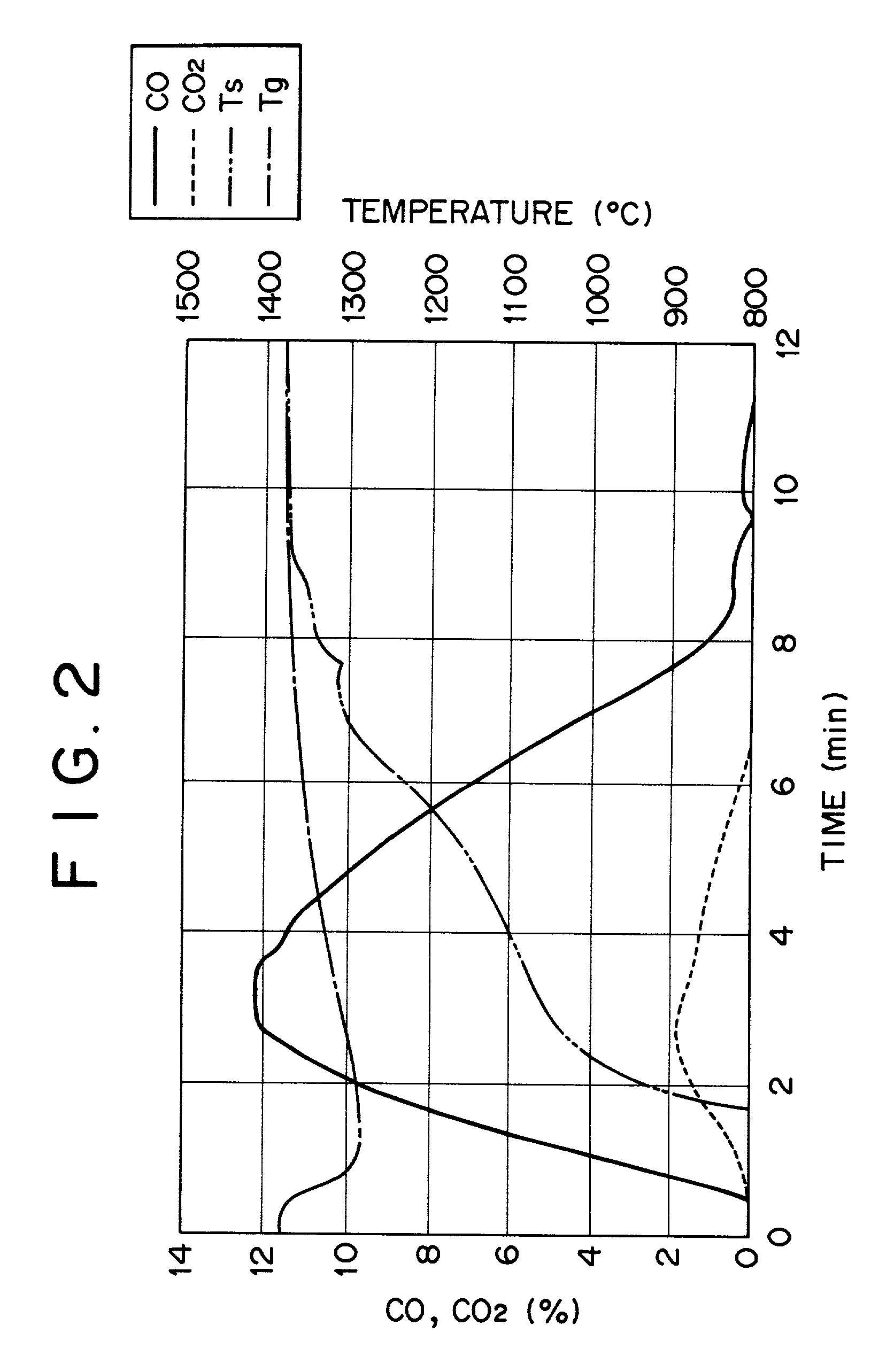
[0078] FIG. 3 shows the relation between the reduction degree of the compact and the temperature of the compact in Example 1. It can be seen from FIG. 3 that the temperature of the compact is from 1000.degree. C. to 1200.degree. C. for the reduction degree of the iron oxide of about from 20% to 85%. In view of the above, it can be seen that even when heat of combustion at high temperature is supplied to the compact, this is consumed by the endothermic reaction, and the temperature of the compact is kept constant and the reaction scheme shown below is established.
[0079] Fe.sub.2O.sub.3+3CO=2Fe+3CO.sub.2
[0080] CO.sub.2+C=2CO
[0081] In the graph, are shown the temperature Ts for the compact and the reduction degree RD for the compact.
example 3
[0082] An experiment was conducted in the same manner as in Example 1 except for changing the atmospheric temperature in the furnace (operation temperature) for 1300.degree. C., 1350.degree. C., 1400.degree. C. and 1450.degree. C. and behavior of the compact was measured. In this experiment, oxygen potential in the compact of 20 to 25 mm diameter was measured by using a needle type oxygen sensor. FIG. 4 shows for a relationship between the temperature and the oxygen potential in the compact on a Fe--O phase diagram.
[0083] As can be seen from FIG. 4, the compact was melted and dripped when the atmospheric temperature in the furnace was 1350.degree. C. or higher but the compact was not melted and dripped when the atmospheric temperature in the furnace was at 1300.degree. C. It can be seen that the compact was melted and dripped when the measured curve remained within the liquid phase existent region (L).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com