Composition and method for removing photoresist and/or resist residue using supercritical fluids
a supercritical fluid and photoresist technology, applied in the direction of detergent compounding agents, cleaning using liquids, instruments, etc., can solve the problems of less desirable plasma etching procedure for resist removal, less visible residues in post-ash plasma etching, etc., and achieve the effect of removing photoresist and/or resist residues from high aspect ratio openings such as submicron grooves, narrow crevices, etc., without damaging the structure being produced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
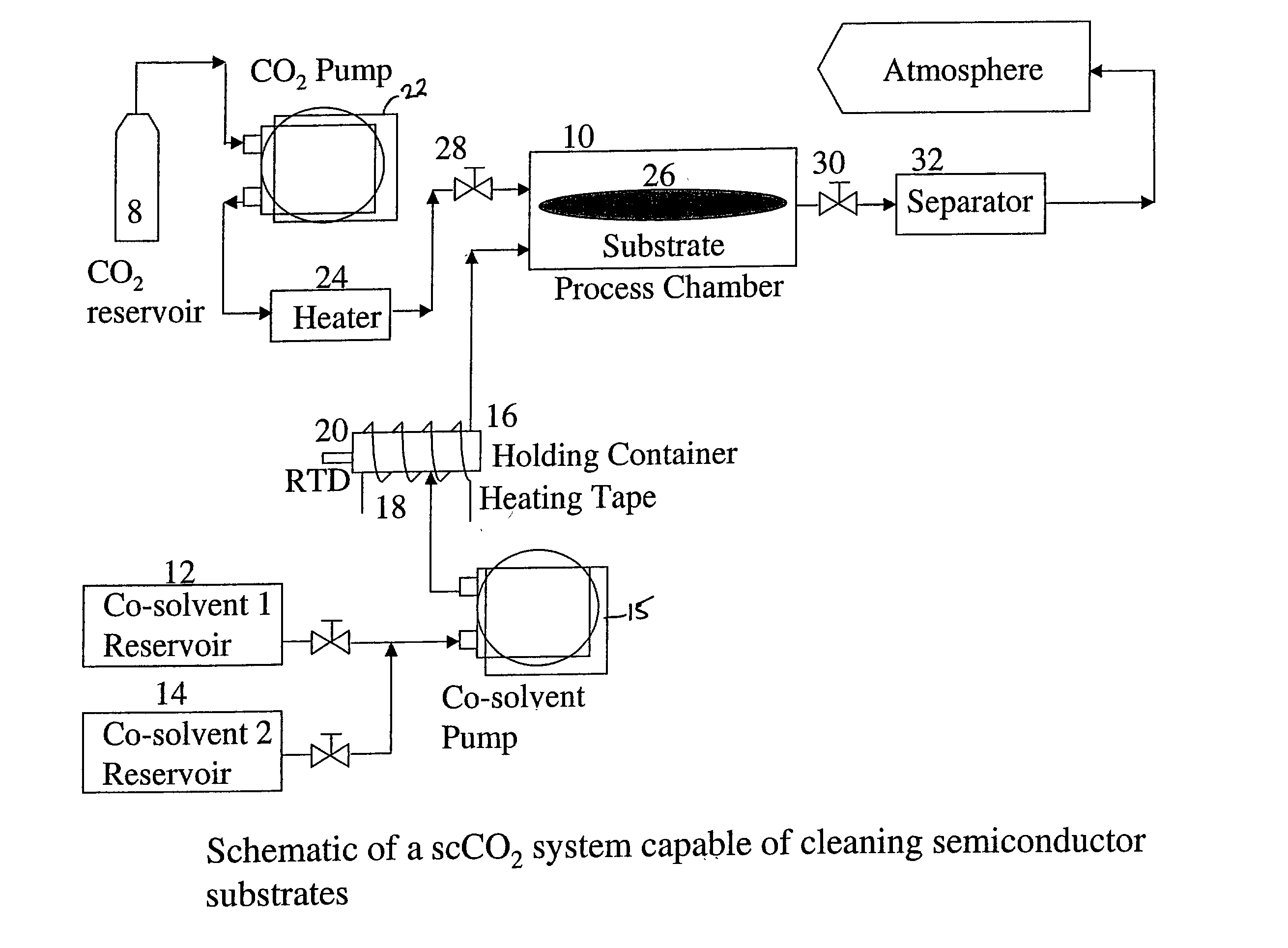
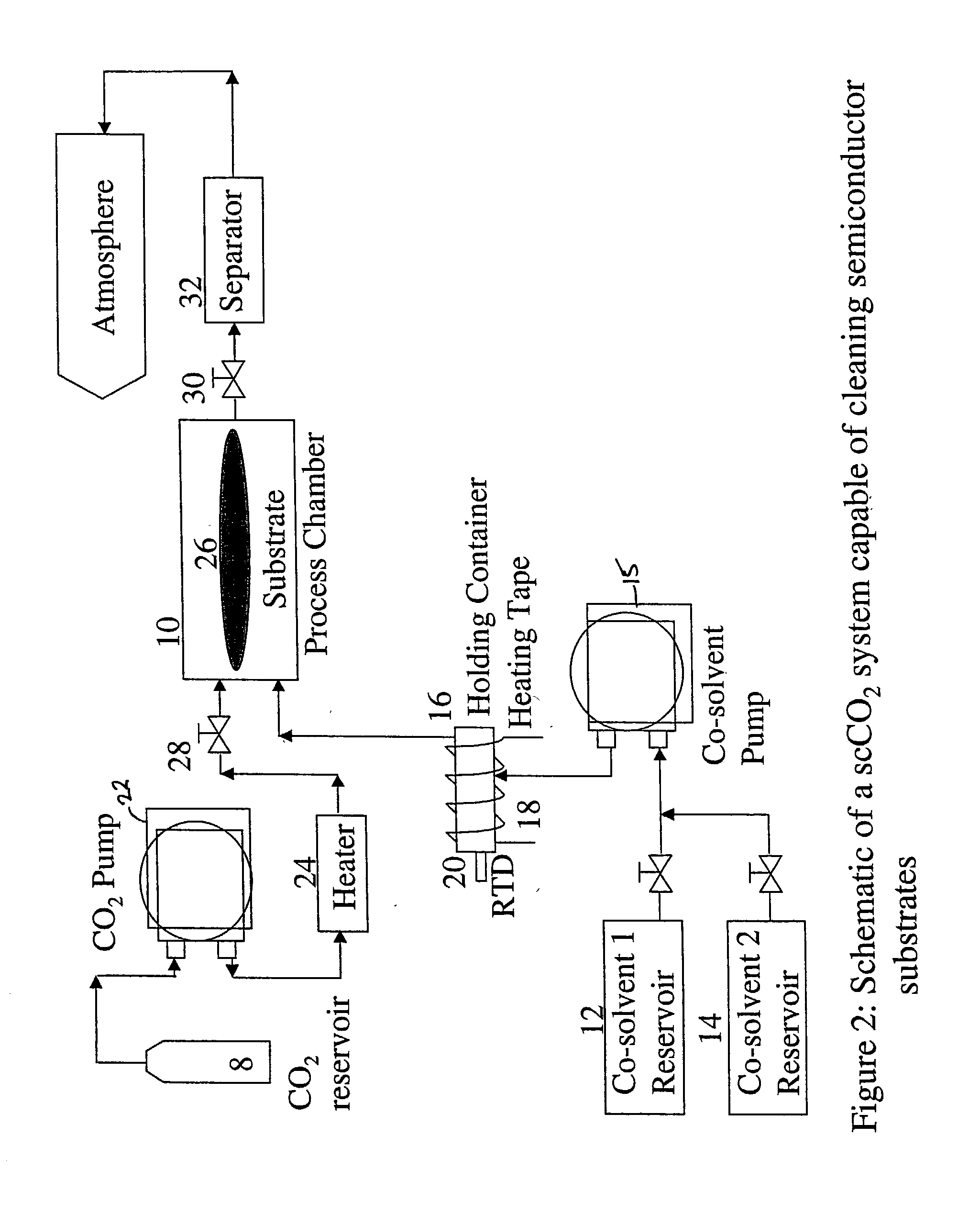
[0056] In the second example, the co-solvent mix is unchanged but is introduced into the process chamber in higher amounts at the start of the run and the complete process is run without any static dwell in the process chamber. A substrate having a hard baked I-line photoresist that was DUV stabilized using UV lamps to achieve 100% cross-linking was placed in the process chamber. A co-solvent I composition of 40% (by weight) 1,2-Butylene Carbonate, 30% Dimethyl Sulfoxide, and 30% of 30% hydrogen peroxide was mixed at a temperature of 50.degree. C. This mixture was made to flow into the process chamber and onto the substrate at a rate of 20 g / min for approximately 30 seconds. Supercritical carbon dioxide was caused to flow into the chamber with the co-solvent 1 at a flow rate of 60 g / min to have a total fluid flow rate into the process chamber at 80 g / min. Subsequently the co-solvent 1 flow rate was decreased to 2.4 g / min and the supercritical carbon dioxide flow rate increased to 77...
example 3
[0059] The third example is similar to Example 2, but differs in that a different cosolvent 1 composition was used. A substrate having a hard baked I-line photoresist that was DUV stabilized using UV lamps to achieve 100% cross-linking was placed in the process chamber. A co-solvent 1 composition of 40% (by weight) 1,2-Butylene Carbonate, 40% Benzyl Alcohol, and 20% of 30% hydrogen peroxide was mixed at a temperature of 50.degree. C. This mixture was made to flow into the process chamber and onto the substrate at a rate of 20 g / min for approximately 45 seconds. Supercritical carbon dioxide was caused to flow into the chamber with the co-solvent 1 at a flow rate of 60. g / min to have a total fluid flow rate into the process chamber at 80 g / min. Subsequently the co-solvent 1 flow rate was decreased to 2.4 g / min and the supercritical carbon dioxide flow rate-increased to 77.6 g / min. for the next 3 minutes and 15 seconds. The operating temperature and pressure within the chamber were 110...
example 4
[0062] The fourth example utilized the same co-solvent 1 composition as used in Example 2, but the composition was used on a substrate having different characteristics. In this example, the blanket photoresist layer removed was a 6000 .ANG. thick DUV 5 photoresist layer on top of a polysilicon layer which covers a silicon dioxide layer on top of the silicon wafer substrate. The photoresist was subjected to a high dose implant of boron at 10 keV to a dosage level of 3.times.10.sup.15 atoms / cm.sup.2. A co-solvent 1 composition of 40% (by weight) 1,2-Butylene Carbonate, 30% Dimethyl Sulfoxide, and 30% of 30% hydrogen peroxide was mixed at a temperature of 50.degree. C. This mixture was made to flow into the process chamber and onto the substrate at a rate of 8 g / min for 4 minutes. The co-solvent 1 mixture was carried into the process chamber by supercritical carbon dioxide at a flow rate of 72 g / min to have a total fluid flow rate into the process chamber at 80 g / min. The operating tem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Dielectric constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com