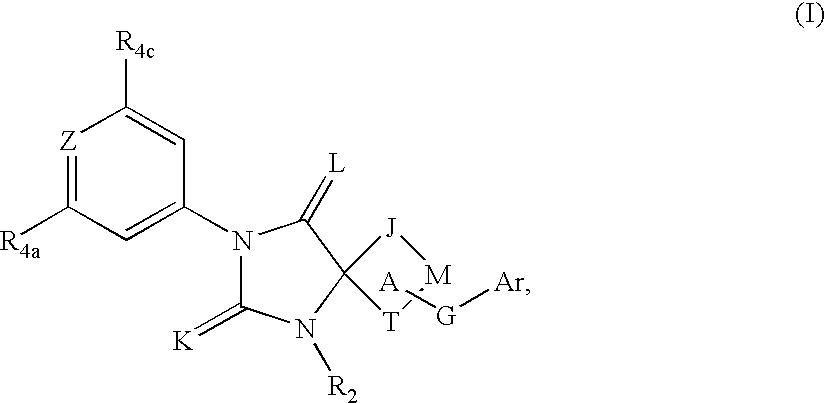
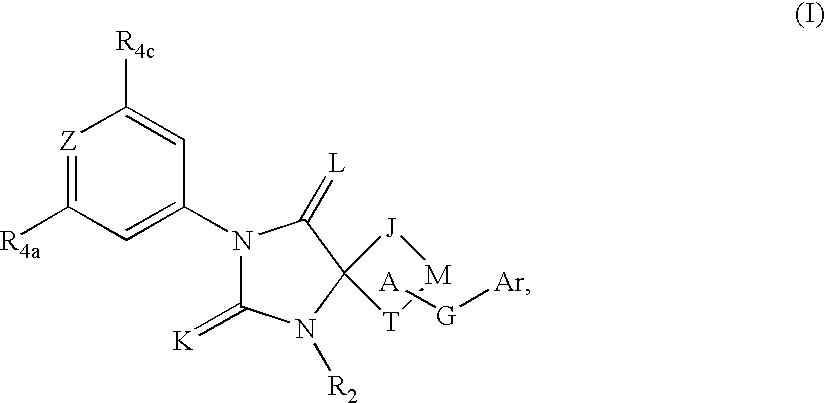
Spiro-hydantoin compounds useful as anti-inflammatory agents
a technology of hydantoin and compounds, which is applied in the field of hydantoin compounds, can solve the problems of chronic inflammation, poor wound healing, and patients' inability to mount a normal inflammatory or immune respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
The following Examples illustrate embodiments of the inventive compounds and starting materials, and are not intended to limit the scope of the claims. For ease of reference, the following abbreviations are used herein:
Abbreviations
AlCl3=aluminum chloride Ac2O=acetic anhydride AcONa=sodium acetate bp=boiling point CH3CN=acetonitrile DCC=dicyclohexylcarbodiimide DCE=dichloroethane DCM=dichloromethane DMAP=4-dimethylaminopyridine DIPEA or DIEA=N,N-diisopropylethylamine DME=1,2-dimethoxyethane DMF=dimethyl formamide EDCI=1-3-dimethylaminopropyl)-3-ethylcarbodiimide Et2O=diethyl ether HOBT=1-hydroxybenzotriazole EtOAc=ethyl acetate EtOH=ethanol g=gram(s) HCl=hydrochloric acid KOH=potassium hydroxide K2CO3=potassium carbonate l=liter LiAlH4=lithium aluminum hydride MeCN=acetonitrile MeOH=methanol MgSO4=magnesium sulfate NaH=sodium hydride Na2SO4=sodium sulfate NaOH=sodium hydroxide NMP=1-methyl-2-pyrrolidinone PBr3=phosphorus tribromide (Ph3P)4Pd=tetrak...
preparation 1
4-(4-Bromophenyl)-4-oxobutyric acid
AlC3 (128.8 g, 0.97 mol) was added by portions within 20 min to a suspension of succinic anhydride (44.3 g, 0.44 mol) and bromobenzene (100 ml, 0.99 mol) in DCM (500 ml) while the reaction flask was cooled in a water bath. After 1h30 min at RT, the reaction mixture was refluxed for 2 h. After cooling, the reaction medium was slowly poured into a mixture of ice (1.5 l) and concentrated HCl (100 ml). The precipitate was washed twice with water, with isopropanol, and finally with pentane. After drying 4-(4-bromophenyl)-4-oxobutyric acid was obtained as an off-white solid (86.4 g, mp=148° C.). 1H NMR (CDCl3): 7.85 (2H, d, J=8.5 Hz), 7.62 (2H, d, J=8.5 Hz), 3.28 (2H, t, J=6.5 Hz), 2.82 (2H, t, J=6.5 Hz).
preparation 2
4-(4-Bromophenyl)-4-oxobutyric acid methyl ester
4-(4-Bromophenyl)-4-oxobutyric acid (86.4 g, 0.336 mol) (Preparation 1) in MeOH (1.7 l) containing H2SO4 (86 ml) was refluxed for 21 h. After cooling, the light precipitate was filtered off and the reaction mixture concentrated to dryness. The obtained solid was placed in water and extracted twice with EtOAc. The organic layer was washed with diluted NaOH and twice with brine, dried over Na2SO4 and concentrated to yield the desired 4-(4-bromophenyl)-4-oxobutyric acid methyl ester as a low melting point solid (87.5 g, mp=50° C.). 1H NMR (CDCl3): 7.85 (2H, d, J=8.5 Hz), 7.60 (2H, d, J=8.5 Hz), 3.71 (3H, s), 3.28 (2H, t, J=6.5 Hz), 2.76 (2H, t, J=6.5 Hz).
PUM
| Property | Measurement | Unit |
|---|---|---|
| adhesive interactions | aaaaa | aaaaa |
| adhesion | aaaaa | aaaaa |
| adhesive function | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com