Helianthrone derivatives as anti-cancer agents
a technology of helianthrone and derivatives, applied in the field of polycyclic dianthraquinones, can solve problems such as severe side effects, and achieve the effects of preventing metastatic growth, preventing incurable metastases, and stimulating the growth of micrometastases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Killing of Malignant Tumor Cells in Culture by Dimethyl-TMH and TMH in the Dark
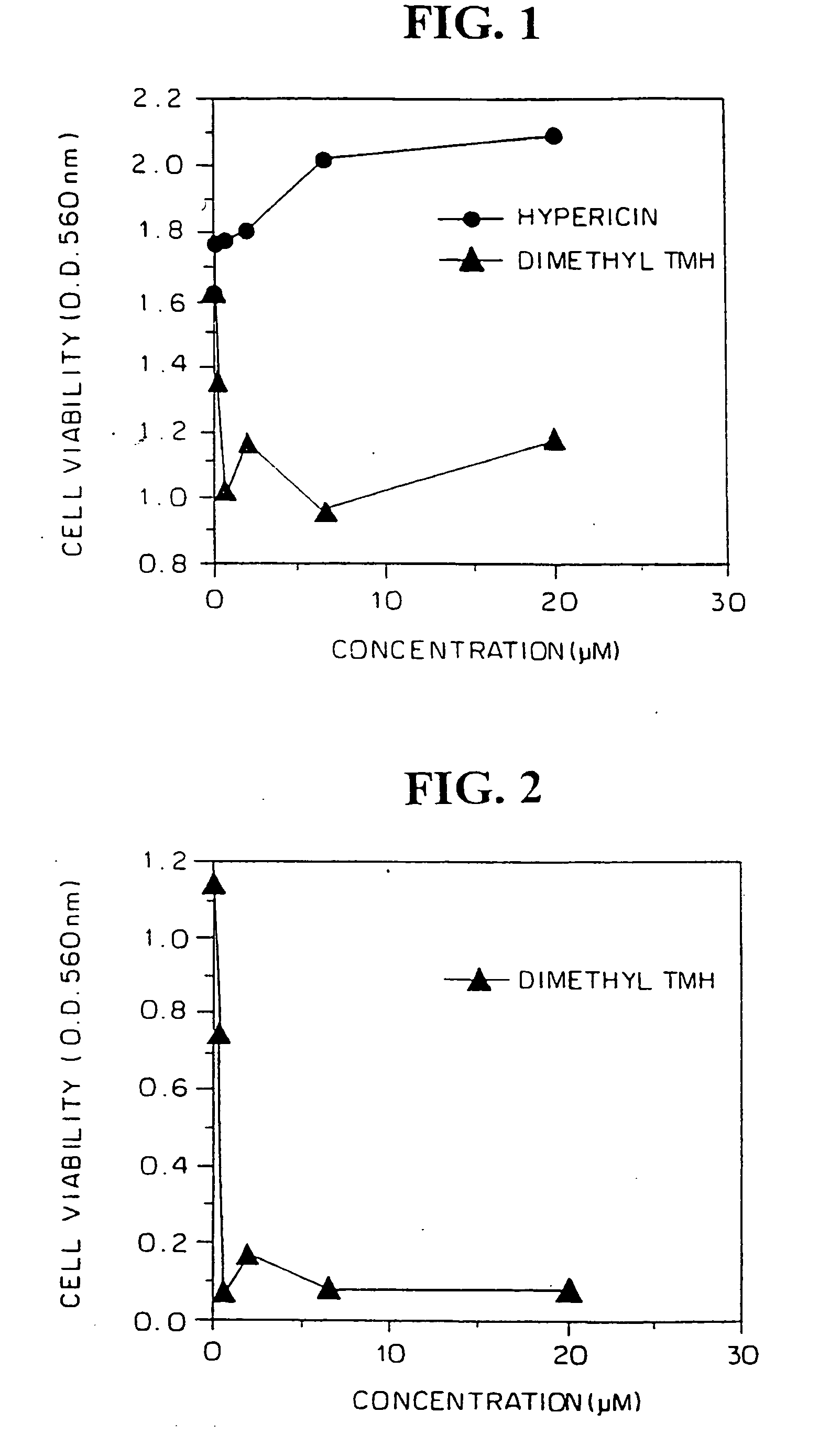
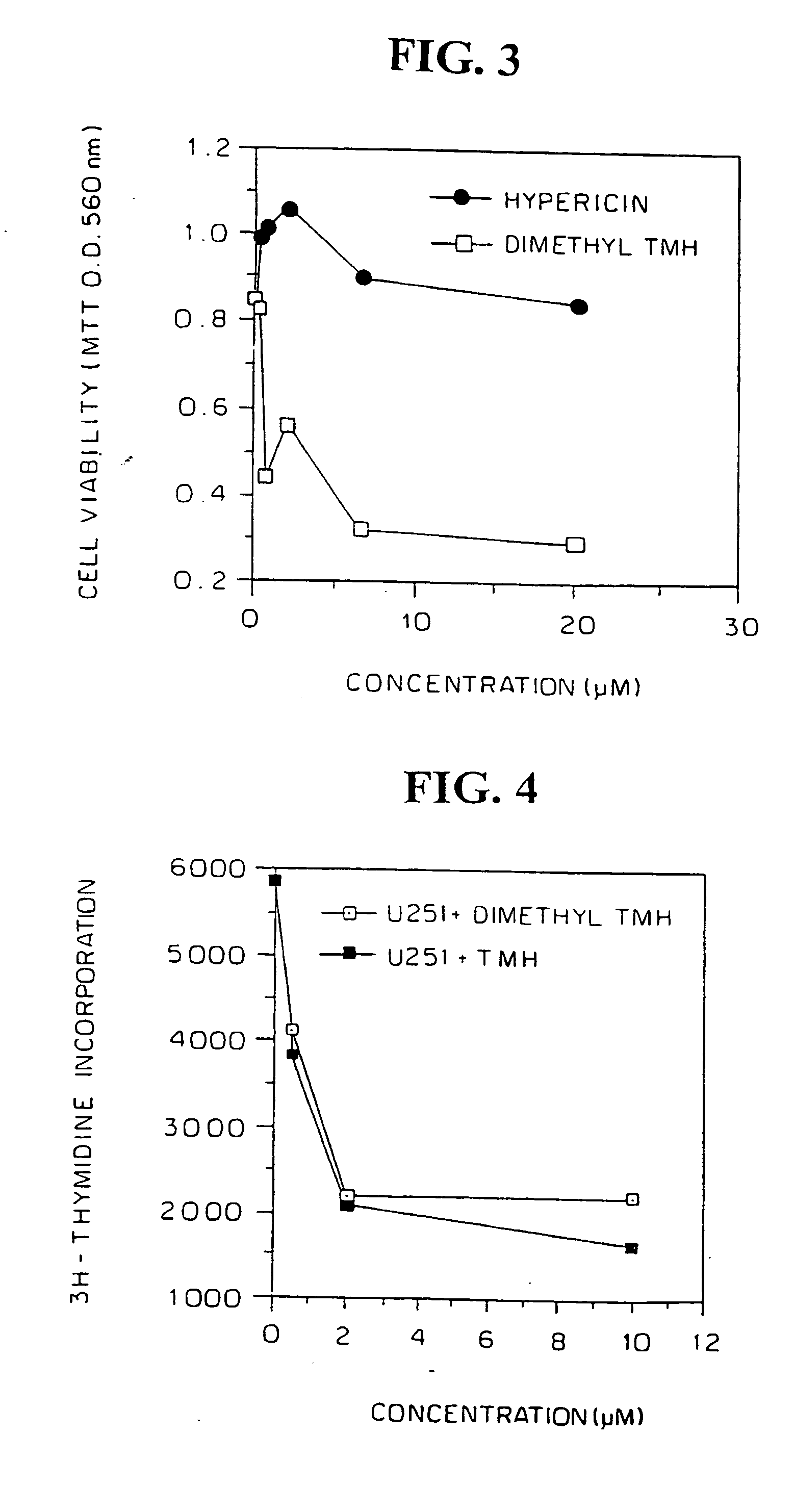
Three human malignant cell lines were evaluated to sensitivity to dimethyl TMH in vitro. Human U251 glioblastoma, U87MG glioblastoma and LAN5 neuroblastoma cells were plated (2×105 per well) in 96-well flat bottom microculture plates, treated with dimethyl-TMH and hypericin at dose ranges of 0 (control), 0.1 -20 μM in complete darkness for a period of 72 hours. The medium was aspirated, the adherent monolayer was washed with phosphate-buffered saline, and cell viability was monitored by the MTT assay.
FIGS. 1, 2 and 3 show the results for the U251 glioblastoma, LAN5 neuroblastoma and U87MG glioblastoma cells, respectively, comparison of the cytotoxic activity with hypericin being shown in FIGS. 1 and 3. Cell viability was lost in all three after exposure to dimethyl-TMH for at least 72 hours, as measured in MTT viability assays. Loss of cell viability following treatment with dimethyl-TMH in the dark of...
example 2
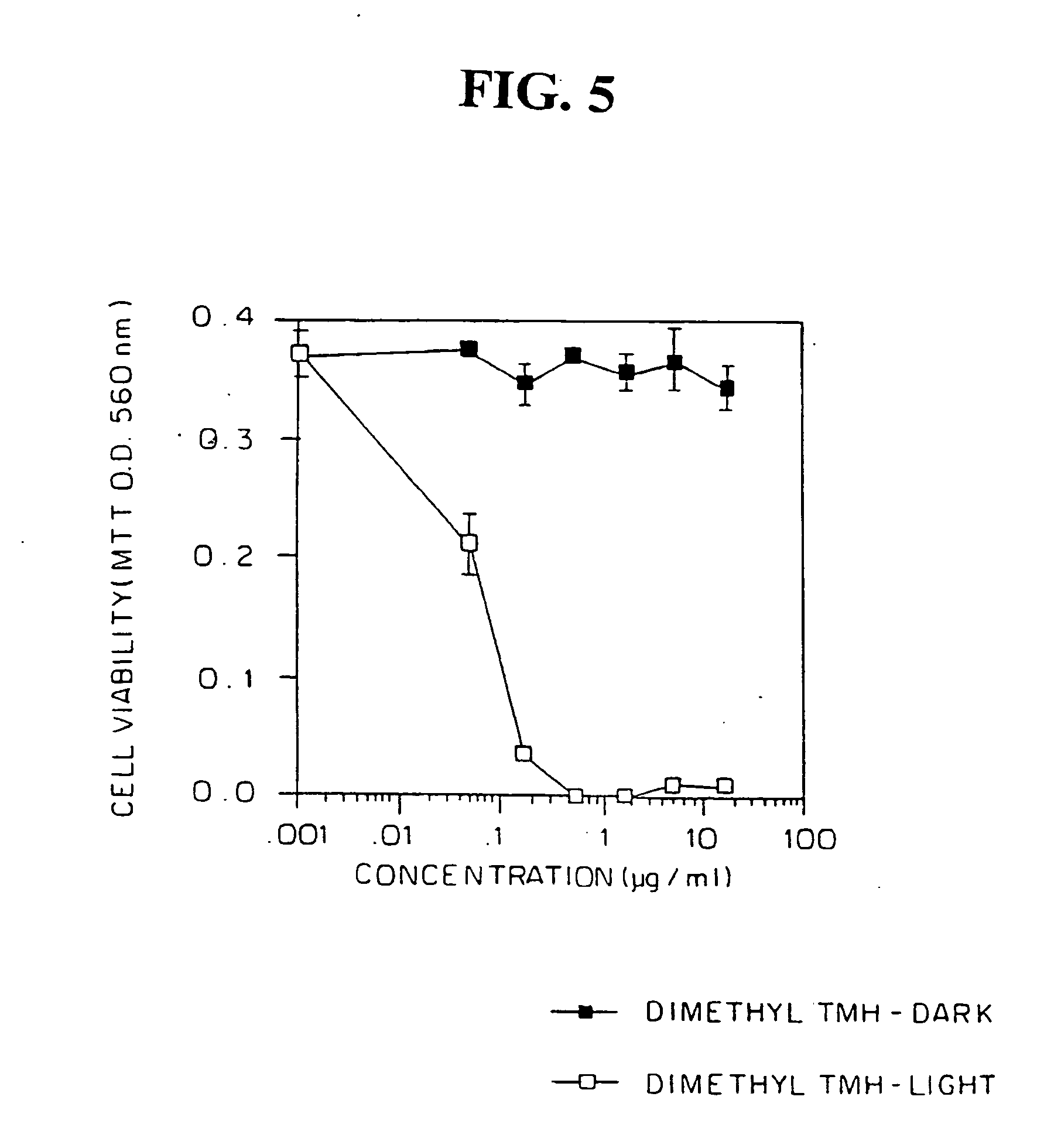
Light-Dependent, Photodynamic Effects of Dimethyl-TMH on Normal Primary Human Peripheral Blood Lymphocytes
Human peripheral blood lymphocytes (PBL) are non-proliferating cells in the absence of mitogenic stimuli. The effects of different doses of dimethyl-TMH on PBL were examined in the presence or absence of irradiation with polychromatic white light. PBL (post-mitotic) were plated (2×105 cells / well) in two separate round bottom 96-well plates (in triplicates). Dimethyl-TMH was added to the cultures. One plate was kept in the dark, and the other was exposed to polychromatic white light at a fluence rate of 8 mW / cm2 for 30 min (a total of 14.4 J / cm2). Both plates were then cultured at 37° C., 5% CO2 for 72 hours and cell viability was assayed by the MTT assay. The results, in FIG. 5, show that dimethyl-TMH had no effect on PBL viability in the absence of light irradiation, however, photosensitization with light caused cell death with an LD50 of approximately 0.65 μM dimethyl TMH, i...
example 3
Determination of the Cell Cycle Phases in which Dimethyl-TMH Arrests Malignant Tumor Cells Growth and Proliferation in the Dark
Cell cycle and DNA content analyses were conducted in U251 human glioblastoma cells after treatment with 5 μg / ml (10 μM) dimethyl-TMH for 24, 48 and 72 hours, and on LAN5 neuroblastoma cells after 48 hours. The cells were then stained with propidium iodide, washed with PBS and analyzed in a fluorescence activated cell sorter (FACS). A computer program arranged the DNA-related fluorescence as follows: the minimal amount of fluorescence is considered to be one whole set of cellular DNA related to the resting G1 phase. A double amount of fluorescence is considered to be G2 phase, in which the whole genome is duplicated following complete DNA synthesis, and the in-between amounts are considered to be the DNA synthetic S-phase, in which the total DNA synthesis is not yet completed.
The results, shown in FIGS. 6-7, reveal that administration of 10 μM dimethyl-T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com