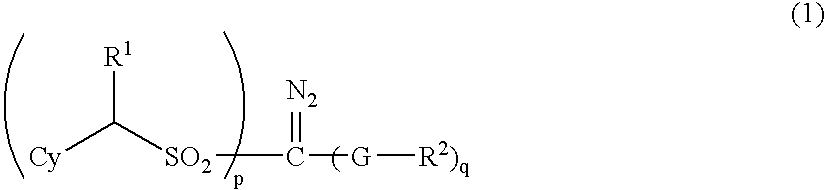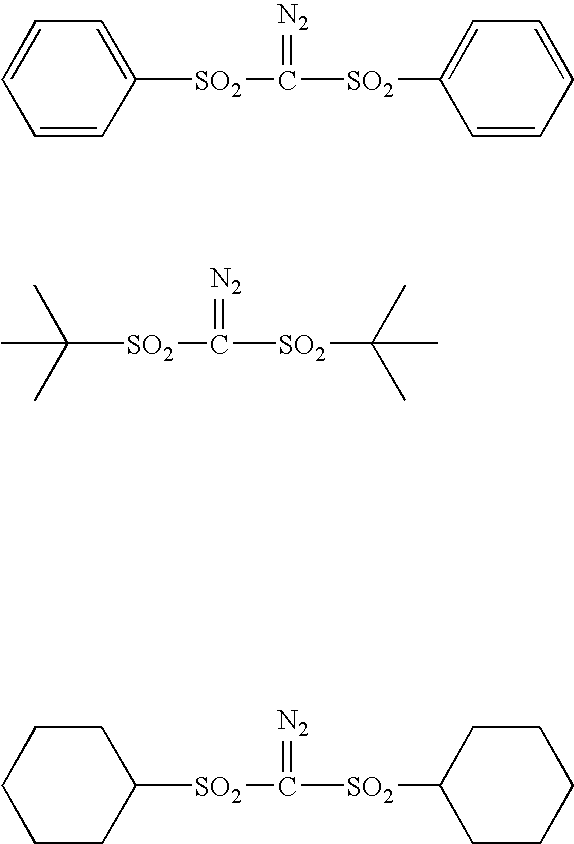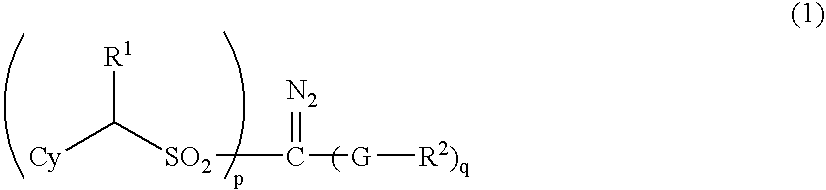Novel sulfonyldiazomethane compounds, photoacid generator, resist materials and patterning process using the same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
Synthesis of ((4-(1,3-dioxolan-2-yl)-1-hydroxycyclohexyl)methyl)trimethylsilane
A Grignard reagent was prepared in a conventional manner by using 44.6 g (0.36 mole) of (chloromethyl)trimethylsilane, 8.8 g (0.36 mole) of metal magnesium and 194 g of diethyl ether. To the resulting Gignard reagent was added 50.9 g (0.33 mole) of 1,4-cyclohexanedione monoketal over an ice bath at a temperature below 20° C. After stirring for 2 hours at 40° C., 125 g of water was added to the resulting mixture over an ice bath. The supernatant thus separated was collected, and washed with 200 g of saturated sodium chloride water. The solvent was removed under reduced pressure, whereby 77.6 g of ((4-(1,3-dioxolan-2-yl)-1-hydroxycyclohexyl)methyl)trimethylsilane was obtained.
synthesis example 2
Synthesis of 1-(1,3-dioxolan-2-yl)-4-methylenecyclohexane
After 15.7 g (0.39 mole) of sodium hydride was washed with n-hexane, it was suspended in 199 g of tetrahydrofuran. A solution obtained by dissolving the above hydroxysilane in 112 g of tetrahydrofuran was added dropwise, followed by aging for 41 hours under heating and reflux. After 140 g of water was added to terminate the reaction, the organic phase was obtained by separation. The resulting organic phase was washed with 60 g of saturated sodium chloride water. The solvent was then removed under reduced pressure, whereby the target 1-(1,3-dioxolan-2-yl)-4-methylenecyclohexane was obtained (two step yield: 77%). Gas chromatography revealed that its purity was 83%.
synthesis example 3
Synthesis of S-((4-(1,3-dioxolan-2-ylcyclohexyl)methyl)thioacetate
In 188 g of tetrahydrofuran was dissolved 46.8 g (0.25 mole) of the above methylenecyclohexane. Thioacetic acid (22.0 ml, 0.31 mole) was added dropwise to the resulting solution at a speed slow enough not to increase the internal temperature, followed by aging for 1 hour. After further addition of 3.5 ml (0.05 mole) of thioacetic acid and aging for 30 minutes, 200 g of a saturated aqueous solution of sodium bicarbonate was added to terminate the reaction. The organic phase obtained by separation of the reaction mixture was washed with 50 g of saturated sodium chloride water. The solvent was then removed under reduced pressure, whereby 76.7 g of the target S-((4-(1,3-dioxolan-2-ylcyclohexyl)methyl)thioacetate was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Nanoscale particle size | aaaaa | aaaaa |
| Solubility (mass) | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com