Therapeutic compounds for inhibiting interleukin-12 signaling and methods for using same
a technology of interleukin-12 signaling and therapeutic compounds, which is applied in the field of therapeutic compounds, can solve the problems of affecting the immune system affecting the survival rate of subjects, etc., and achieves the effect of limiting the inflammatory response of subjects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
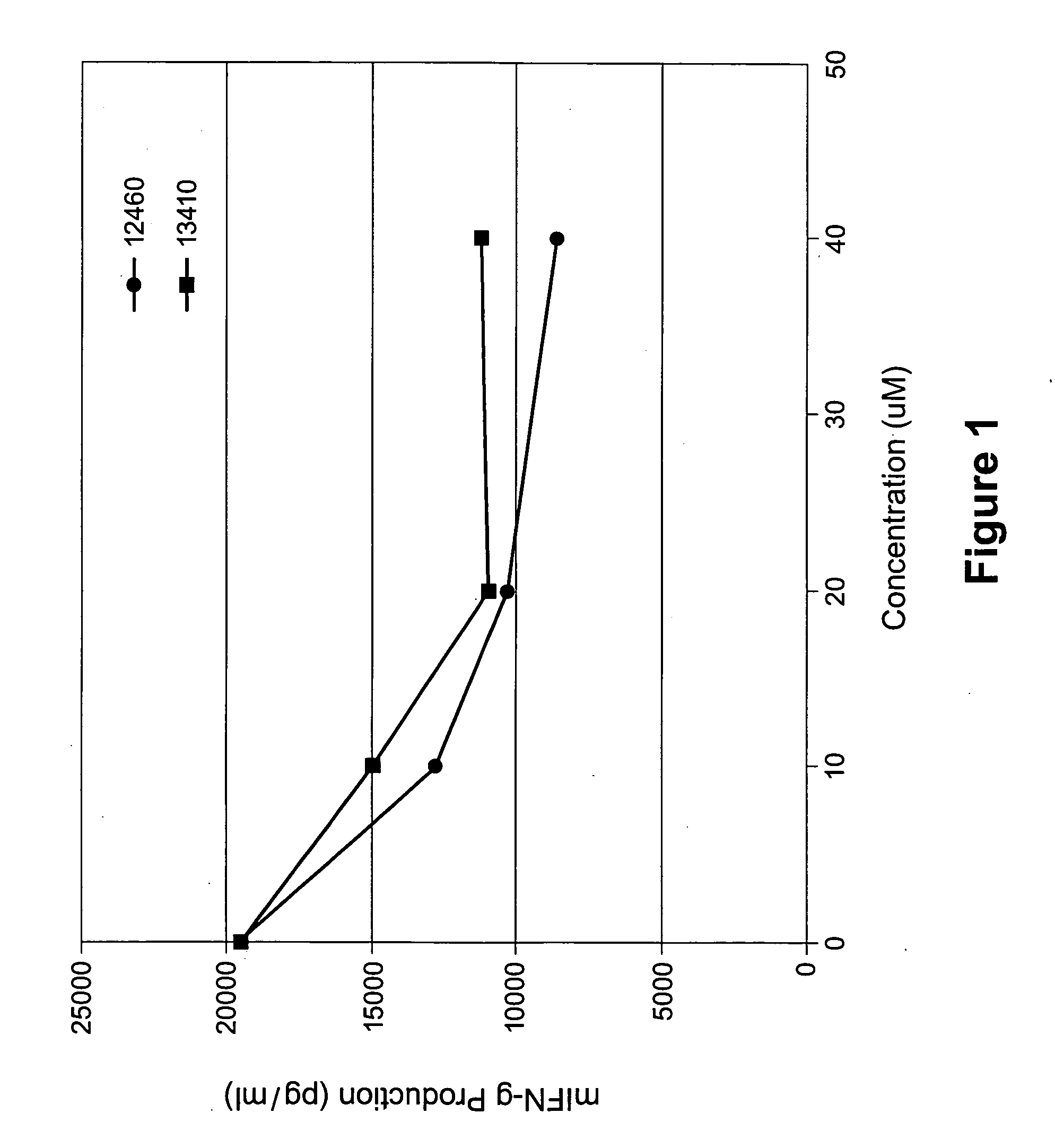
Image
Examples
example 1
Synthesis of 1-(5-(N-Hydroxy)aminohexyl)-3,7-dimethylxanthine (CT7549)
[0160] Sodium cyanoborohydride (62.84 mg, 1 mmol) was added to a solution of 1-(5-oximinohexyl)-3,7-dimethylxanthine (Klein, J. P.; Leigh, A. Oxime Substituted Therapeutic Compounds, U.S. Pat. No. 5,770,595 (Jun. 23, 1998)) (293 mg, 1 mmol) in methanol (10 ml). 1 M hydrogen chloride in ether was added to pH 4-5. After stirring for 3 hours, the mixture was concentrated under reduced pressure. 1 N aqueous sodium hydroxide solution to pH 9-10 (10 ml). The mixture was extracted with 10% methanol-dichloromethane (3×50 ml). The combined extracts were washed with water (50 ml), dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The residue was purified by flash chromatography on silica gel eluting with 10% methanol-dichloromethane to provide 1-(5-(N-hydroxy)aminohexyl)-3,7-dimethylxanthine (180 mg).
example 2
Synthesis of (R)-3-(5-Hydroxyhexyl)-1,7-dimethylxanthine (CT11495)
[0161] To a stirring solution of 1,7-dimethylxanthine (0.30 g, 1.67 mmol) in dimethylsulfoxide (20 ml) was added sodium hydride (42 mmg, 1.75 mmol) in one portion. After stirring for 30 minutes, (R)-5-acetoxy-1-bromohexane (0.31 g, 1.75 mmol) was added neat. (R)-5-Acetoxy-1-bromohexane was prepared according to methods described in U.S. patent application Ser. No. 09 / 002,345, which is incorporated herein by reference. After heating at 80° C. for 18 hours, water (25 ml) was added and the aqueous solution was extracted with dichloromethane (3×20 ml). The combined extracts were washed with saturated aqueous sodium chloride solution (50 ml), dried over sodium sulfate and concentrated under reduced pressure. The residue was purified by flash chromatography on silica eluting with ethyl acetate to give (R)-3-(5-acetoxyhexyl)-1,7-dimethylxanthine (0.34 g, 64% yield) as a colorless oil.
[0162] To a stirring solution of (R)-3-...
example 3
Synthesis of (R)-1-(5-Hydroxyhexyl)-3,7-dimethyluric acid (CT11499)
[0163] To a stirring solution of 3,7-dimethyluric acid (0.40 g, 2.04 mmol) in dimethylsulfoxide (20 ml) was added sodium hydride (49 mg, 2.04 mmol) in one portion. After stirring for 45 minutes, a solution of chloromethyl pivalate (0.29 g, 2.04 mmol) in dimethylsulfoxide (1 ml). After stirring for 24 hours, water (50 ml) was added. After cooling to room temperature, the solid was filtered, rinsed with water (4×20 ml), with ether (20 ml) and dried under vacuum to give 9-pivaloyloxymethyl-3,7-dimethyluric acid (0.18 g, 28% yield) as a white solid.
[0164] To a stirring solution of 9-pivaloyloxymethyl-3,7-dimethyluric acid (0.14 g, 0.45 mmol) in dimethylsulfoxide (10 ml) was added sodium hydride (12 mg, 0.47 mmol) in one portion. The solution was stirred for 15 minutes. (R)-5-Acetoxy-1-iodohexane (0.13 g, 0.47 mmol) in dimethylsulfoxide (1 ml) was added. The solution of (R)-5-acetoxy-1-iodohexane was prepared according ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com