Methods of prevention and treatment using apolipoprotein analogues
a technology of apolipoprotein and analogues, applied in the field of prevention and treatment using apolipoprotein analogues, can solve the problems of increasing the risk of infarction, and achieve the effects of reducing the rate of filtration through the kidneys, reducing the half-life and increasing the size of the apolipoprotein constru
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
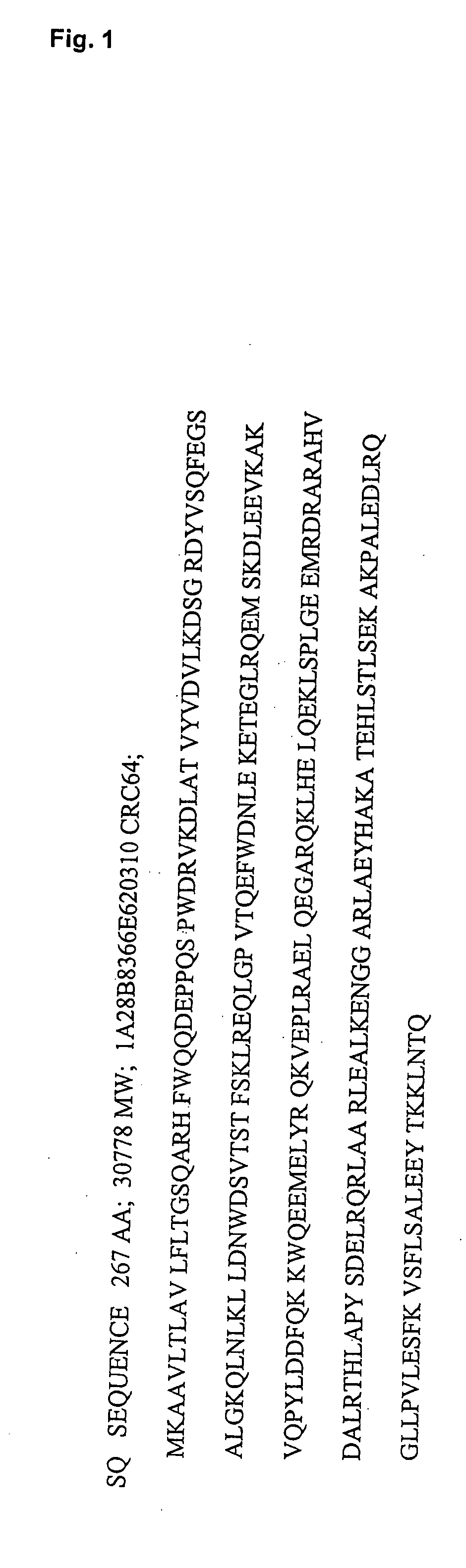
Cloning of Apo A-I
[0229] The cDNA encoding Apo A-I was amplified from a human liver cDNA library (Clontech) using standard PCR techniques. For the construction of Ubi-A-I the primers used were: 5′-CAC GGA TCC ATC GAG GGT AGG GGT GGA GAT GAA CCC CCC CAG AGC-3′ and 5′-TCC AAG CTT ATT ACT GGG TGT TGA GCT TCT TAG TG-3′. The product was cloned into the vector pT7H6Ubi, described in (Ellgaard L. et al Eur. J. Biochem. 1997; 244(2):544-51) using the Bam HI and Hind III cloning sites. For the construction of Trip-A-A-I the primers used were 5′-AAG GGA TCC GAT GAA CCC CCC CAG AGC CCC-3′ and 5′-TCC AAG CTT ATT ACT GGG TGT TGA GCT TCT TAG TG-3′. The PCR product was cloned into the pT7H6tripa vector described in WO 98 / 56906 using the Bam HI and Hind III cloning sites. For the construction of Trip-A-I-del43 the primers used were 5′-AGG GGA TCC CTA AAG CTC CTT GAC AAC TGG G-3′ and 5′-TCC AAG CTT ATT ACT GGG TGT TGA GCT TCT TAG TG -3′. The PCR product was cloned into the pT7H6tripa vector describ...
example 2
Expression of Apolipoprotein A-I (Apo A-I) in E. coli
[0230] Ubi-A-I and Trip-A-I as well as the other constructs disclosed in the figures are conveniently expressed in E. coli AV-1 cells (Stratagene Inc.). Other cell lines may be used as well. Culturing of the cells and induction of expression were performed as described for tetranectin in WO 98 / 56906.
example 3
Isolation and Processing of Protein
[0231] Crude protein was isolated by phenol extraction as described for tetranectin in WO 98 / 56906. The re-dissolved pellet from 6 litres of expression culture was centrifuged to remove non-dissolved material and then batch adsorbed to 50 ml Ni2+-NTA-Sepharose, prepared as described in WO 98 / 56906. The column material was packed on a column and then washed with 500 ml 8 M urea, 500 mM NaCl, 50 mM Tris-HCl pH 8.0, then 200 ml of 6 M Guanidinium-HCl, 50 mM Tris-HCl pH 8.0 and finally 300 ml of 500 mM NaCl, 50 mM Tris-HCl pH 8.0. The protein was eluted with 500 mM NaCl, 50 mM Tris-HCl pH 8.0 and 10 mM EDTA. The protein was added 0.5 mg of Factor Xa and digested overnight at room temperature. Thrombin may be used for this purpose as well. The protein was gelfiltrated on a G-25 sephadex (Pharmacia) column in to a 500 mM NaCl, 50 mM Tris-HCl pH 8.0 buffer. Undigested protein was removed by passing the protein solution over a Ni2+-NTA-Sepharose column pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dissociation constant | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com