Oral insulin therapies and protocol
a technology of oral insulin and insulin therapy, which is applied in the direction of peptide/protein ingredients, extracellular fluid disorder, metabolic disorder, etc., can solve the problems of limited use of biological macromolecules as active agents in pharmaceutical compositions, many obstacles to successful oral delivery of biological macromolecules, and primarily limited expression of glucokinas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
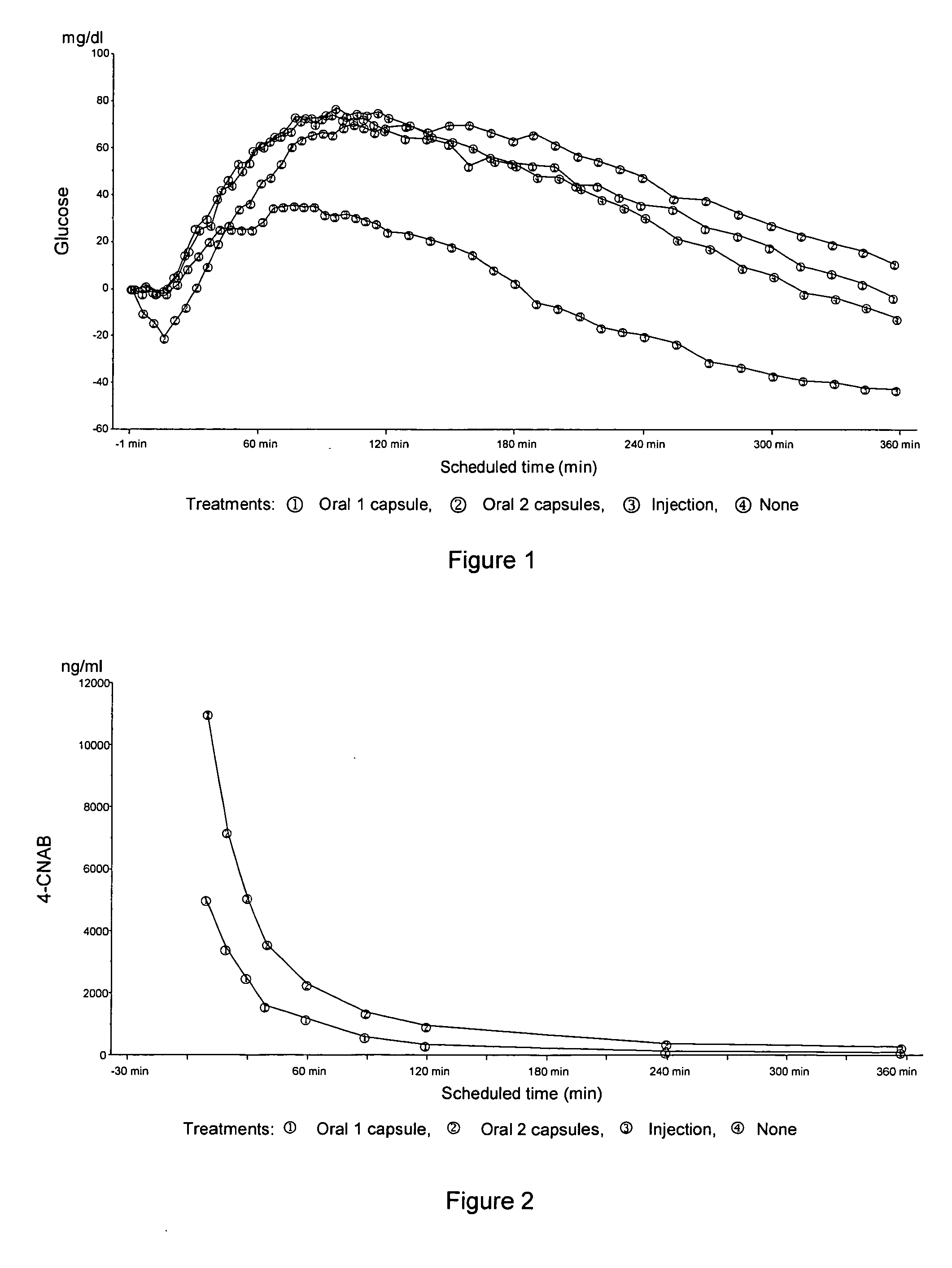
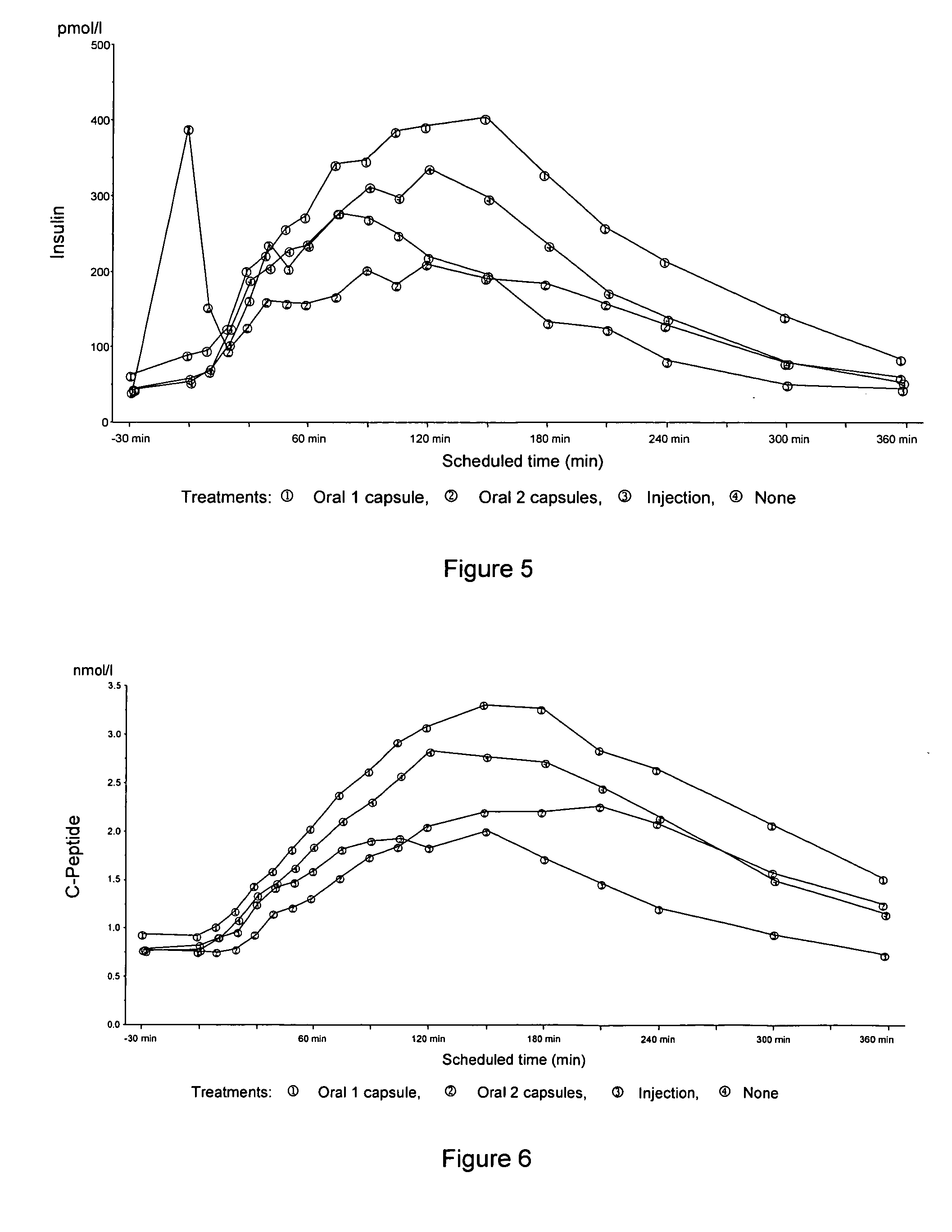
Comparison Between Oral Insulin and SC Short Acting Postprandial Blood Glucose Excursions
[0295] A randomized, 3-period crossover, double-blind, double-dummy study was conducted in order to compare the effect (i.e., the postprandial pharmacokinetic and pharmacodynamic profiles) of an oral insulin formulation with that of subcutaneously administered short acting insulin on postprandial blood glucose excursions in type 2 diabetic subjects without any antidiabetic medication.
[0296] A primary objective of this study was to compare the effect of an oral insulin formulation (300 U insulin combined with 400 mg 4-CNAB in 2 capsules, each capsule containing 150 U insulin / 200 mg 4-CNAB) with that of 12 U subcutaneous (SC) injected short acting insulin [Humalog® injection 100 U / ml from Eli Lilly and Company] on postprandial blood glucose excursions. The postprandial blood glucose excursions were assessed after a standardized breakfast intake.
[0297] Fifteen male subjects between 35 and 70 ye...
example 2
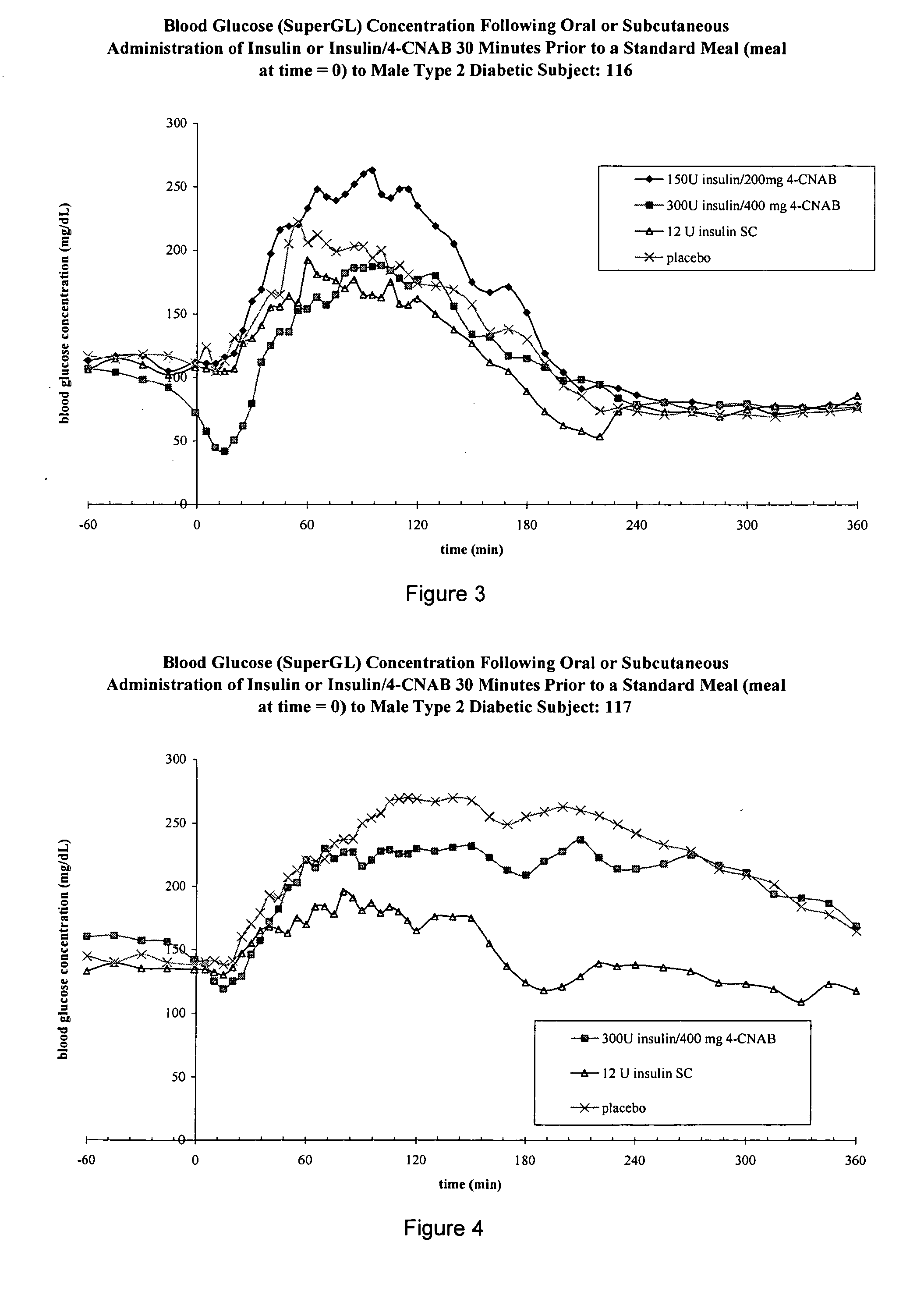
[0416] In this example, as also set forth in International Patent Application No. PCT / US04 / 00273, oral insulin capsule(s) described herein were orally administered to twenty human subjects with diabetes at night before going to sleep.
[0417] An open-label, single-dose, crossover study was conducted in order to compare the safety of orally administered 4-CNAB / Insulin formulation with that of subcutaneously injected insulin in two groups of subjects with type 2 diabetes mellitus—one in the fasting state and one after a standard meal. The objectives were (1) to compare the safety, pharmacokinetics and pharmacodynamics of orally administered 4-CNAB / insulin with that of subcutaneously injected regular insulin in fasting type 2 diabetic subjects, and (2) to compare blood glucose, insulin and C-peptide levels after a standard meal with regular medication with blood glucose, insulin and C-peptide levels after a standard meal with 4-CNAB / insulin.
[0418] The focus of this study is the assessm...
example 3
Preparation of Insulin / 4-CNAB (75 U / 100 mg) Tablets
[0431] This example describes the manufacturing procedure for Insulin / 4-CNAB tablets. Each tablet contained about 75 units of insulin USP (equivalent to about 2.82 mg of recombinant human insulin with an as-is potency of about 26.6 U / mg) and about 100 mg of 4-CNAB monosodium salt.
[0432] Composition of Formulation (Theoretical all Numbers are Approximate):
ComponentWeight (mg) / tablet4-CNAB, monosodium salt100Insulin2.82Povidone0.41Anhydrous Emcompress45.27(extragranular)Magnesium Stearate (extragranular)1.5Total150
[0433] 4-CNAB and povidone (KOLLIDON® 90F (BASF Corporation, Mount Olive, N.J.)) were weighed. KOLLIDON® 90F was dissolved in 15% w / w water. Insulin (obtained from Diosynth, Inc.) was suspended in the KOLLIDON® solution, and then 4-CNAB was granulated using the insulin suspension as granulation media. Granulation was completed with additional water, as required. Granules were dried in a vacuum oven at about 50° C. Partl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com