Compositions for enhancing biological functions in organisms
a biological function and composition technology, applied in the field of compositions for enhancing biological functions in organisms, can solve the problems of complex induced resistance systems in plants, damage to fungal hosts, and inability to obtain nutrients, so as to improve the growth of fungal cells and enhance the production of extracellular enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Remote Sensing by Elicitors
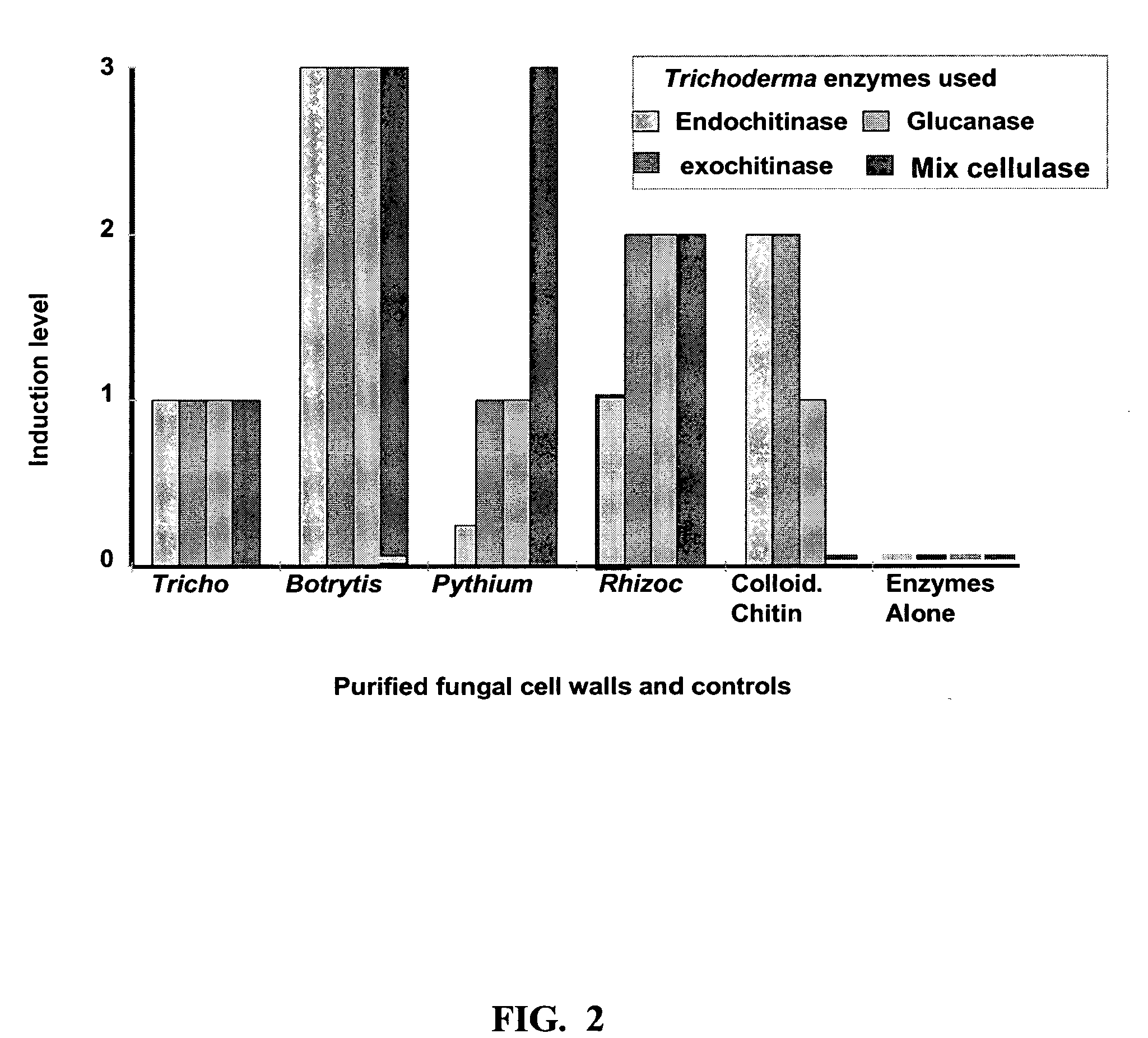
[0061] The discovery of the eliciting compounds of the present invention occurred primarily by defining the remote sensing system describe herein above. Past research demonstrated (a) that Trichoderma strains sense fungi that it can parasitize at a distance and grow tropically toward the target fungus (Chet et al., “Trichoderma Hamatum: Its Hyphal Interactions with Rhizoctonia Solani and Pythium spp,”Microb Ecol 7:29-38 (1981), which is hereby incorporated by reference in its entirety) and (b) that endochitinase, but not exochitinase (N-acetylglucosaminidase) is stimulated to be produced by Trichoderma spp. prior to contact with the fungus (Zeilinger et al., “Chitinase Gene Expression During Mycoparasitic Interaction of Trichoderma Harzianum with its Host,”Fung Genet Biol 26:131 -140 (1999), which is hereby incorporated by reference in its entirety). Both enzymes are important in the parasitic process along with various glucanases and other enzymes and an...
example 2
Sources and Release of Elicitors
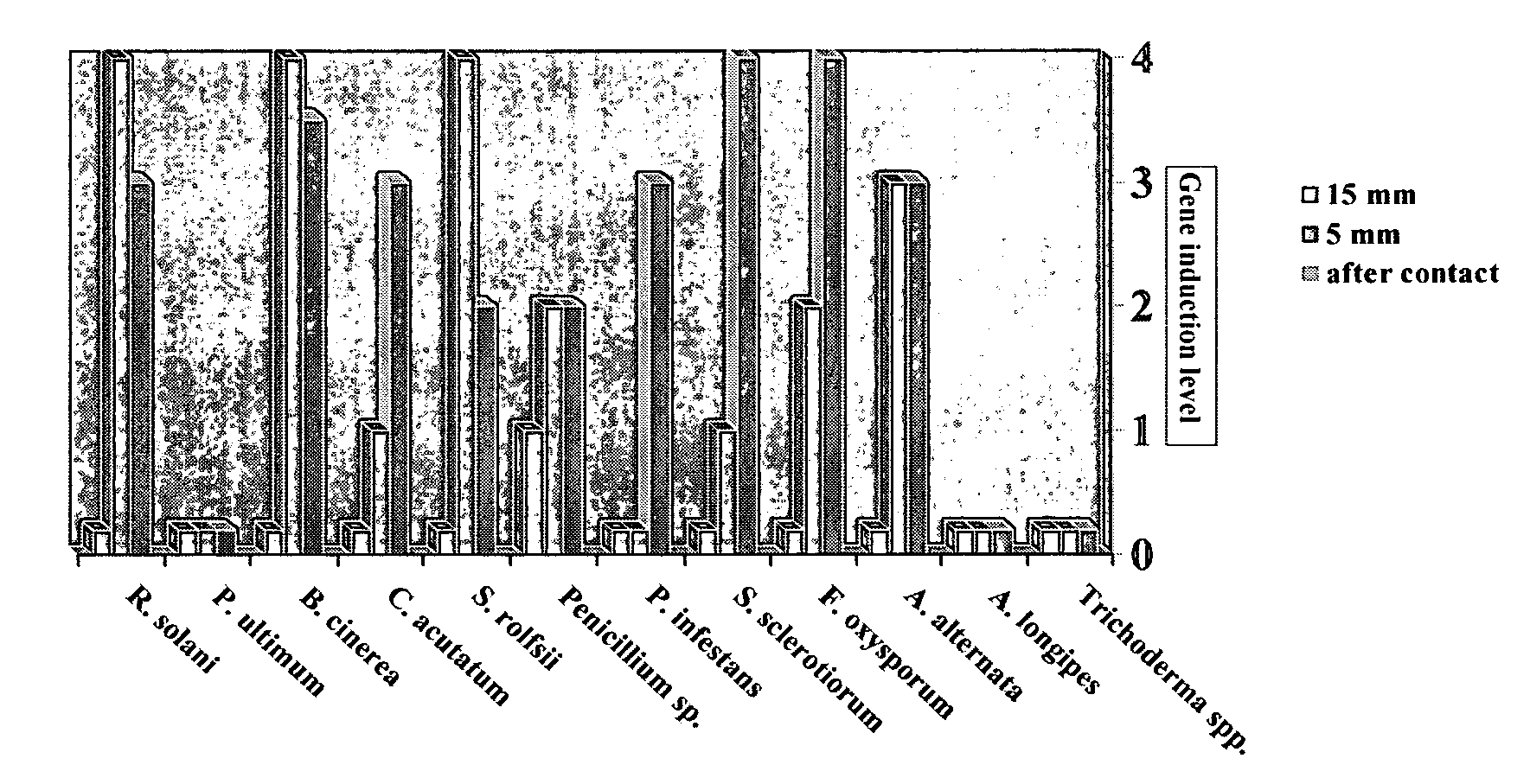
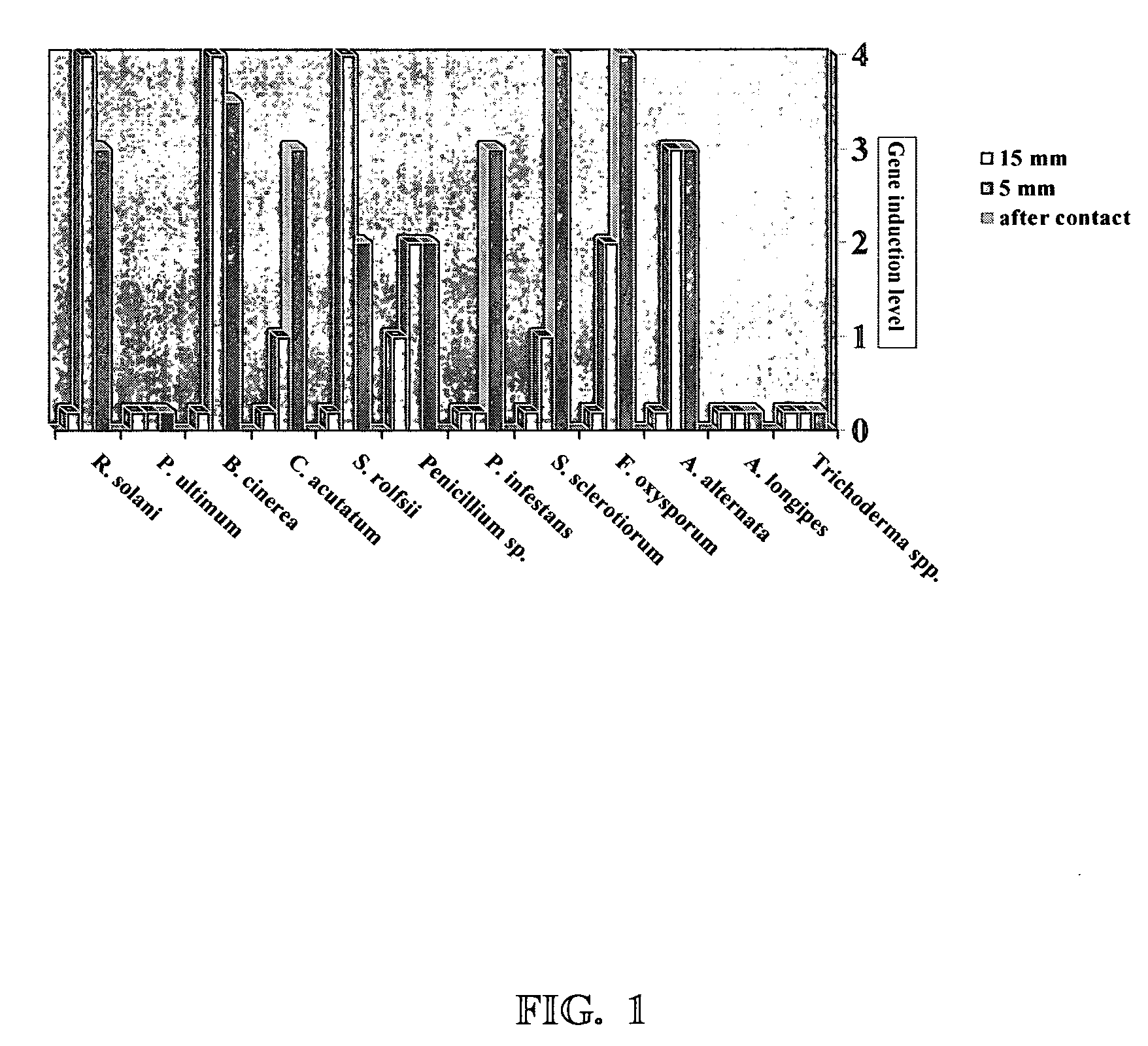
[0065] The ability of Trichoderma to release elicitor compounds from the various target fungi was measured by induction of gfp in the endochitinase promoter labeled P1 strain of Trichoderma. Trichoderma strain P1, transformed to contain an endochitinase promoter:gfp construct, was placed 15 mm or 5 mm distance from the elicitor compound source organism, and, finally in direct contact with various fungi and filamentous Oomycetes. The results are shown in FIG. 1.
[0066] In FIG. 1, the level of endochitinase induction is shown on the right hand axis. Gfp gene induction is measured as intensity of gfp fluorescence produced, with 0 being no fluorescence and 4 being the highest level of fluorescence. The graph provides information regarding the distance between the Trichoderma and the target fungus when gene induction (gfp fluorescence) was measured. The elicitor source organisms are shown on the horizontal axis. R(hizoctonia) solani and S(clerotinia) rolf...
example 3
Identification of the Elicitor Compounds
[0069] Samples with known elicitor activity were fractionated to contain only those compounds with a mass of 3 kDa or less. Compounds in these samples were subjected to high performance liquid chromatography and fractions with elicitor activity, measured with the T. atroviride:gfp system described in Example 1, were identified. These active fractions were then subjected to electrospray mass spectrometry and compounds were identified. FIG. 3 shows the active fractions and their identity. Compounds with elicitor activity are listed in herein above. In summary, the active molecules can be described generally as follows: [0070] (diacetylchitobiose)n, where n=1-5, and having either no additional amino acid moiety, or having one or more amino acid moieties (valine, L-ornthine, and / or serine) associated with, or attached thereto. [0071] 2. (diacetylchitobiose)n, having a (dimeric hexose)n, associated with or attached thereto, where n=1-5, and having...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com