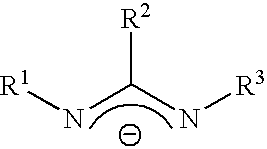
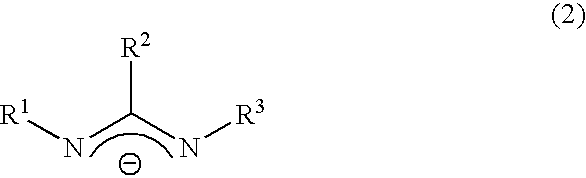
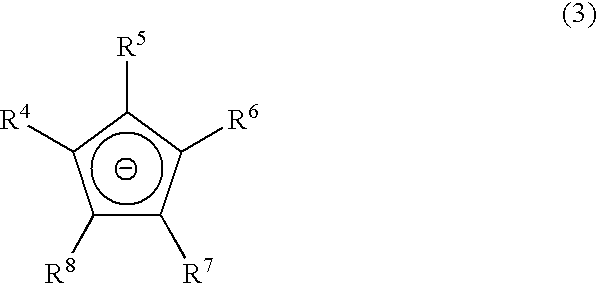
Chemical vapor deposition material and chemical vapor deposition
a technology of chemical vapor and material, applied in the field of chemical vapor deposition material and chemical vapor deposition, can solve the problems of reducing the conductivity of the memory cell, affecting the quality of ruthenium, and affecting the quality of ruthenium, and achieve the effect of high-quality ruthenium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthetic example 1
[0043] 5.7 g of N,N′-diisopropylacetamidine was weighed and placed in a 200 ml flask whose inside had been substituted by nitrogen and left at 50° C. under reduced pressure for 60 minutes. After the inside temperature of the flask was returned to room temperature, the flask was filled with dry nitrogen. 50 ml of well dried diethyl ether was added to the flask in a nitrogen atmosphere and stirred to dissolve the above N,N′-diisopropylacetamidine. This solution was cooled to −60° C., and 22 ml of a diethyl ether solution of butyl lithium (concentration of 2.0 mol / l) was added dropwise under agitation over 30 minutes and further stirred for another 3 hours. Agitation was stopped, the inside temperature of the flask was returned to room temperature over 2 hours, and the supernatant was collected with a syringe to obtain a diethyl ether solution of a lithium salt of N,N′-diisopropylacetamidine.
[0044] Meanwhile, 2.1 g of anhydrous ruthenium trichloride was weighed and placed in a 200 ml ...
synthetic example 2
[0046] 2.1 g of N,N′-diisopropylacetamidine was weighed and placed in a 100 ml flask whose inside had been substituted by nitrogen and left at 50° C. under reduced pressure for 60 minutes. After the inside temperature of the flask was returned to room temperature, the flask was filled with dry nitrogen. 20 ml of well dried diethyl ether was added to the flask in a nitrogen atmosphere and stirred to dissolve the above N,N′-diisopropylacetamidine. This solution was cooled to −60° C., and 9 ml of a diethyl ether solution of butyl lithium (concentration of 2.0 mol / l) was added dropwise under agitation over 30 minutes and further stirred for another 3 hours. Agitation was stopped, the inside temperature of the flask was returned to room temperature over 2 hours, and the supernatant was collected with a syringe to obtain a diethyl ether solution of a lithium salt of N,N′-diisopropylacetamidine.
[0047] Meanwhile, 2.1 g of anhydrous ruthenium trichloride was weighed and placed in a 200 ml f...
synthetic example 3
[0049] 6.8 g of N,N′-di-t-butylacetamidine was weighed and placed in a 200 ml flask whose inside had been substituted by nitrogen and left at 50° C. under reduced pressure for 60 minutes. After the inside temperature of the flask was returned to room temperature, the flask was filled with dry nitrogen. 50 ml of well dried diethyl ether was added to the flask in a nitrogen atmosphere and stirred to dissolve the above N,N′-di-t-butylacetamidine. This solution was cooled to −60° C., 22 ml of a diethyl ether solution of butyl lithium (concentration of 2.0 mol / l) was added dropwise under agitation over 30 minutes and further stirred for another 3 hours. Agitation was stopped, the inside temperature of the flask was returned to room temperature over 2 hours, and the supernatant was collected with a syringe to obtain a diethyl ether solution of a lithium salt of N,N′-di-t-butylacetamidine.
[0050] Meanwhile, 2.1 g of anhydrous ruthenium trichloride was weighed and placed in a 200 ml flask w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com