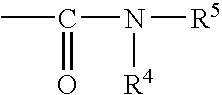
Fluoropolymer and composition thereof
a technology of fluororesin and polymer, applied in the field of fluororesin, can solve the problems of poor moldability, workability, mechanical characteristics and creep characteristics, inferior from the economic viewpoint, and high cost of fluororesin, and achieve the effects of high moldability, stress cracking resistance, and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
Synthesis of a Carbonate Group-Containing, Fluorine-Containing Cohesive Ethylenic Polymer (F-A)
[0202] A 1,280-L autoclave was charged with 380 L of distilled water and, after thorough nitrogen substitution, further charged with 84 kg of perfluorocyclobutane, 166 kg of hexafluoropropylene and 0.5 kg of perfluoro(1,1,5-trihydro-1-pentene), and the system inside was maintained at 26° C. and at a rate of stirring of 200 rpm. Then, tetrafluoroethylene was charged into the autoclave under pressure until 0.86 MPa and, further, ethylene was charged thereinto until 0.92 MPa. The system inside temperature was raised to 35° C., followed by addition of 8.5 kg of a 50% methanol solution of di-n-propyl peroxydicarbonate to initiate the polymerization reaction. The system inside pressure was maintained at 1.15 MPa by continuously feeding a mixed gas composed of tetrafluoroethylene, ethylene and hexafluoropropylene in the mole percentage ratio of 41.0:44.0:15.0, since otherwise the system inside p...
synthesis example 2
Synthesis of a Carbonate Group-Containing, Fluorine-Containing Cohesive Ethylenic Polymer (F-B)
[0205] The same autoclave as used in Synthesis Example 1 was charged with 380 L of distilled water and, after thorough nitrogen substitution, further charged with 166 kg of perfluorocyclobutane, 84 kg of hexafluoropropylene and 0.3 kg of perfluoro(1,1,5-trihydro-1-pentene), and the system inside was maintained at 35° C. and at a rate of stirring of 200 rpm. Then, tetrafluoroethylene was charged into the autoclave under pressure until 0.88 MPa and, further, ethylene was charged thereinto until 0.94 MPa, followed by further addition of 9.0 kg of a 50% methanol solution of di-n-propyl peroxydicarbonate to initiate the polymerization reaction. The system inside pressure was maintained at 0.94 MPa by continuously feeding a mixed gas composed of tetrafluoroethylene, ethylene and hexafluoropropylene in the mole percentage ratio of 46.0:44.0:10.0, since otherwise the system inside pressure would ...
synthesis example 3
Synthesis of a Carbonate Group-Containing, Fluorine-Containing Cohesive Ethylenic Polymer (F-C)
[0207] The same autoclave as used in Synthesis Example 1 was charged with 380 L of distilled water and, after thorough nitrogen substitution, further charged with 230 kg of perfluorocyclobutane and 0.9 kg of perfluoro(1,1,5-trihydro-1-pentene), and the system inside was maintained at 20° C. and at a rate of stirring of 200 rpm. Then, tetrafluoroethylene was charged into the autoclave under pressure until 0.78 MPa and, further, ethylene was charged thereinto until 0.89 MPa and, after raising the system inside temperature to 35° C., 1.1 kg of cyclohexane was added, followed by addition of 1.6 kg of a 50% methanol solution of di-n-propyl peroxydicarbonate to initiate the polymerization reaction. The system inside pressure was maintained at 1.20 MPa by continuously feeding a mixed gas composed of tetrafluoroethylene and ethylene in the mole percentage ratio of 57:43, since otherwise the syste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com