Pharmaceutical composition containing decoy and use of the same
a technology of pharmaceutical compositions and compound compounds, applied in drug compositions, immunological disorders, cardiovascular disorders, etc., can solve the problems of difficult elimination of risk factors, substantial fatality of aortic aneurysmal rupture, and difficulty in clinical applications for treatment (therapy and prevention) of various diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Overexpression of ets-1 in Human Aortic Aneurysm Sample
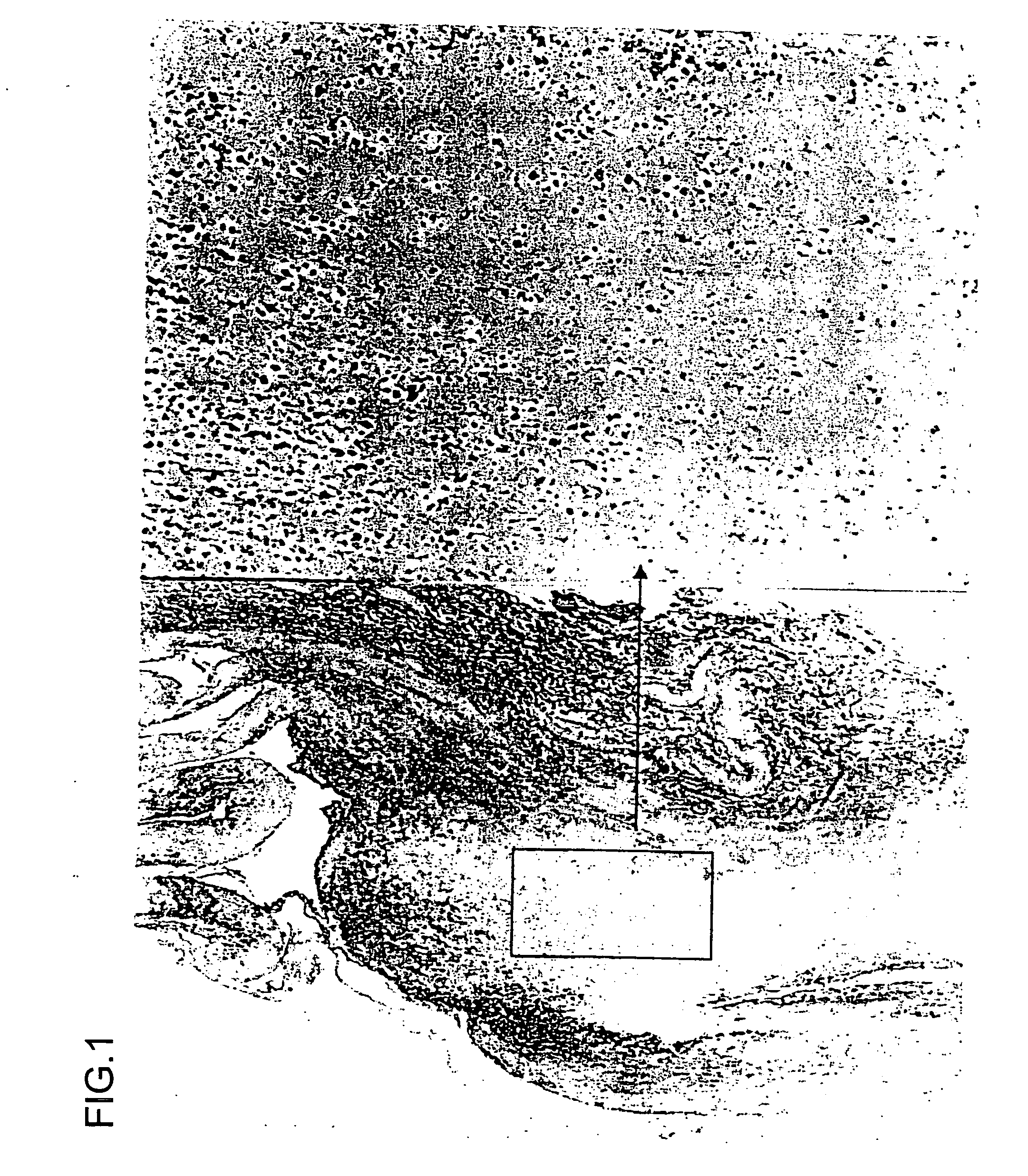
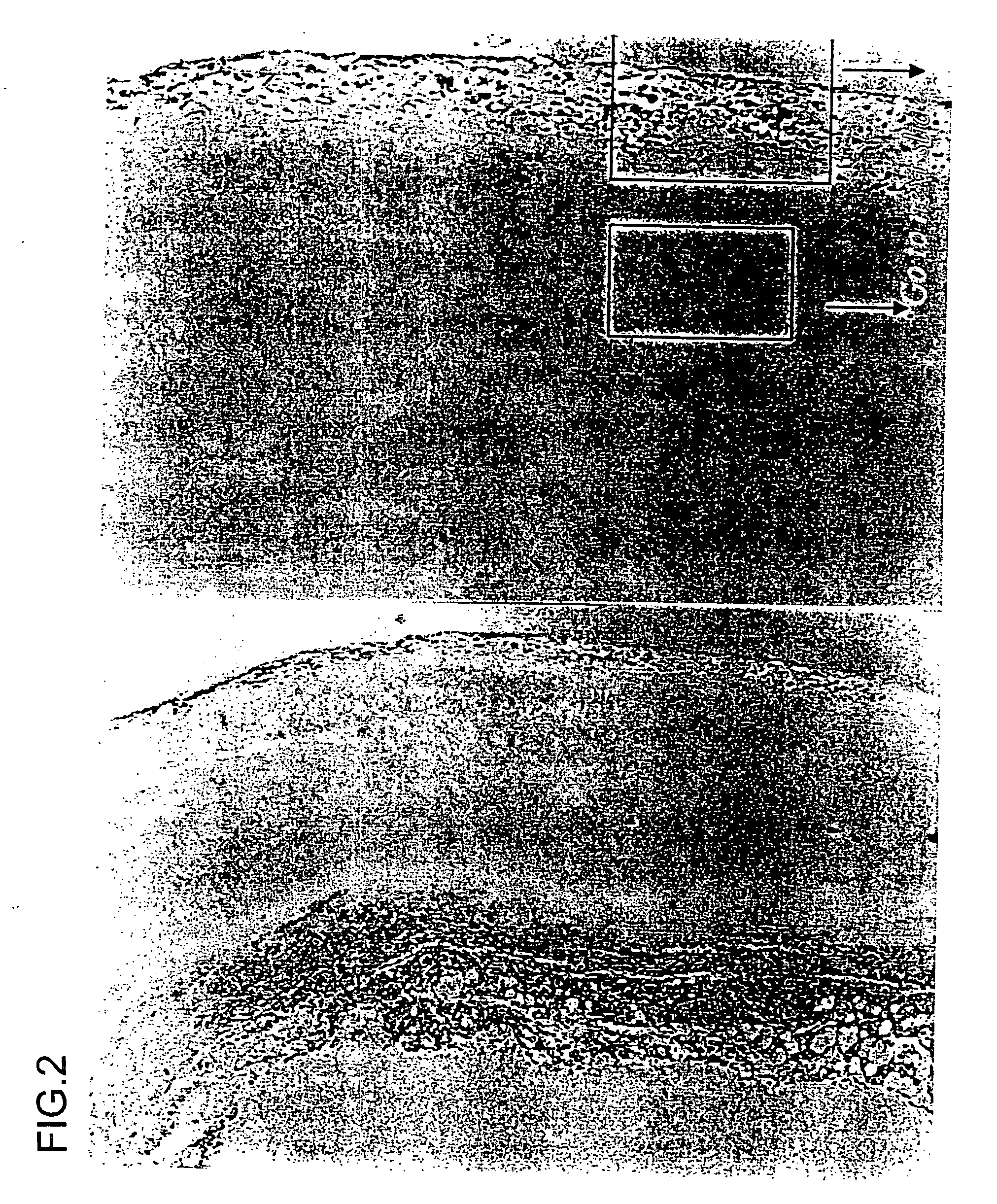
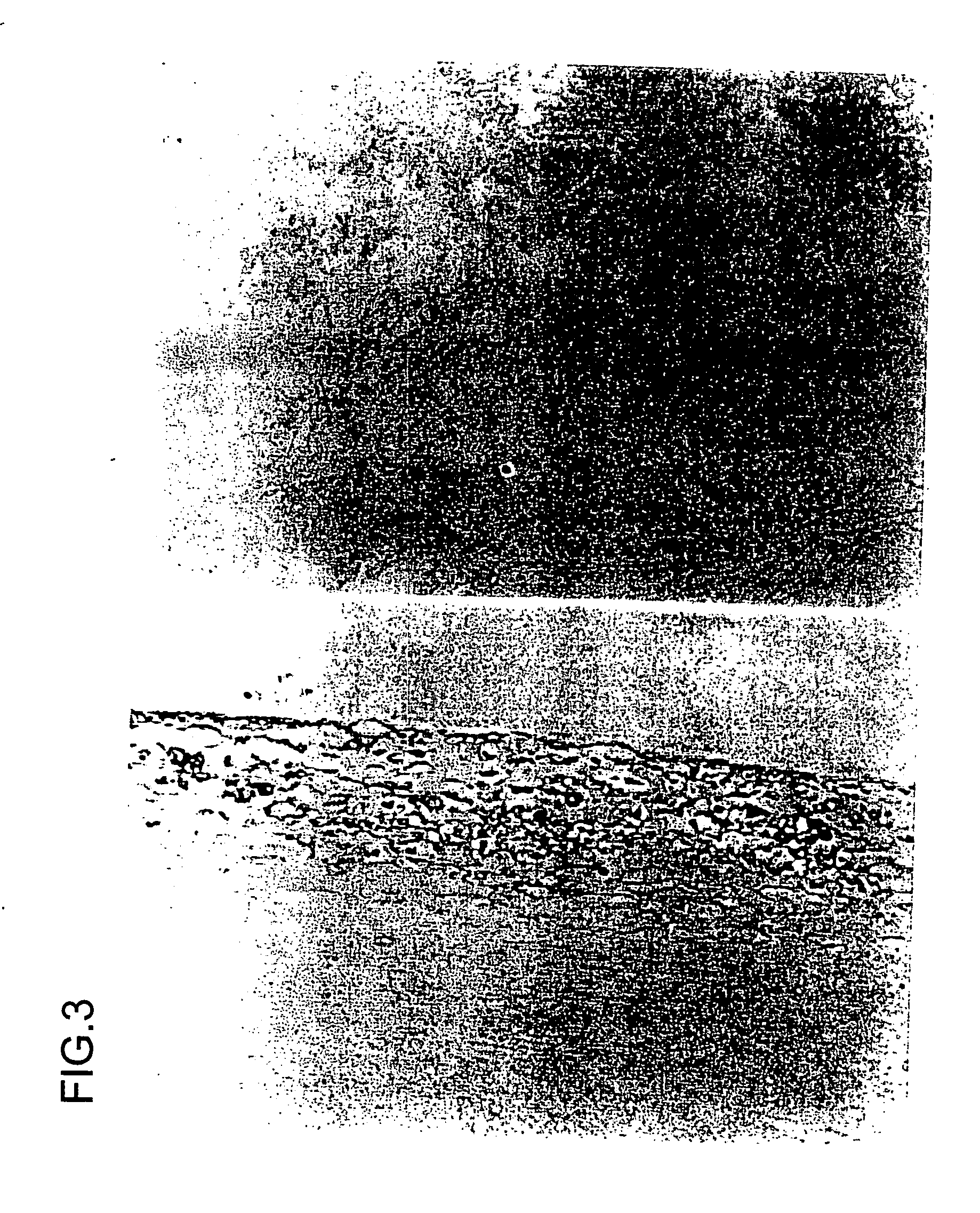
[0119] Ets-1 is one of the transcriptional factors which regulate expression of the MMP gene. Aortic aneurysm samples were surgically removed (excised) and fixed in formalin. The samples were subjected to commonly used immunostaining using an anti-ets-1 antibody (available from Santa Cruz Biotechnology (USA)). As shown in FIGS. 1, 2, and 3, the presence of ets-1 was confirmed in all of the aortic aneurysm samples, mainly the adventitia thereof.
[0120] A photograph to the left of FIG. 1 is a light micrograph showing the human aortic root (×100). A photograph to the right of FIG. 1 is an enlarged photograph (×400) of a rectangular section in the left photograph.
[0121] A photograph to the left of FIG. 2 is a light micrograph showing the human aortic root (×100).
[0122] A photograph to the left of FIG. 2 is a light micrograph (×100) of the most expanded portion of the human aorta. A photograph to the right of FIG. 2 is a fluoresce...
example 2
Effect of Decoy Nucleic Acid in Organ Culture (Tissue Culture)
[0124] Aortic aneurysm samples surgically removed were used in organ culture (tissue culture) to test an effect of decoy nucleic acid transfer on suppression of MMP gene expression.
[0125] Human aortic aneurysm was surgically removed and divided into 2 mm2 samples. The samples were immersed in 10% collagen gel containing 100 μM of a decoy or a scrambled decoy (synthesized by Hokkaido System Science) at room temperature for 1 hour. Thereafter, the samples were placed in 24-well plates with the gel being attached to the samples. 1.5 ml of culture medium (Dulbecco's modified Eagle's medium, 1% FCS) was added to each well, followed by culturing at 37° C. in an incubator. After 24 hours, the culture medium was removed and new culture medium was added to the plate. After another 48 hours, MMP1 and MMP9 in the culture medium were measured by a commonly used method using ELISA (manufactured by Amersham Pharmacia Biotech).
[0126]...
example 3
Concentration-Dependent Effect of Decoy Nucleic Acid and Double Decoy Nucleic Acid on Organ Culture (Tissue Culture System)
[0129] An effect of decoy nucleic acid addition on suppression of MMP gene expression was tested in organ culture (tissue culture system) by the same method as in Example 2, except that the added decoy nucleic acids were 100 μM and 600 μM NF-κB decoy, and 100 μM and 600 μM double decoy and double scrambled decoy having the following structure.
Double decoy5′-ACC-GGA-AGT-AGA-AGG-GAT-TTC-CCT-(SEQ ID NO. 5)CC-3′3′-TGG-CCT-TCA-TCT-TCC-CTA-AAG-GGA-GG-5′Double scrambled decoy5′-GCA-ACC-CCT-TAG-GTT-CTG-AGA-GAC-(SEQ ID NO. 6)GA-3′3′-CGT-TGG-GGA-ATC-CAA-GAC-TCT-CTG-CT-5′
[0130] The results are shown in FIGS. 6 and 7. In FIGS. 6 and 7, the vertical axis represents absorbance at 450 nm, while “untreat”, “NFsd”, “NF100”, “NF600”, “DD sd”, “DD100”, and “DD600” on the horizontal axis represent no nucleic acid reagent (control), 100 μM NF-κB decoy, 600 μM NF-κB decoy, double ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Pharmaceutically acceptable | aaaaa | aaaaa |
| Hydrophilicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com