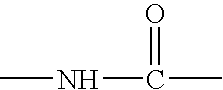
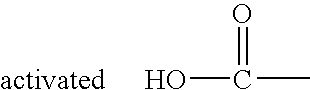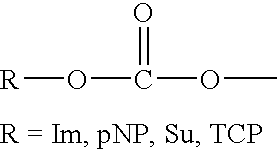Solid phase method for synthesis peptide-spacer-lipid conjugates, conjugates synthesized thereby and targeted liposomes containing the same
a peptide-spacer and conjugate technology, applied in the field of solid phase synthesis of peptide-spacerlipid conjugates, can solve the problems of antibody-based targeted liposomes not using the endocytosis pathway into the interior of cells by the antigen on the cell membrane, and the toxicity of normal tissues, so as to achieve the effect of reducing product loss during synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Amino Alcohol
[0192] Preparation of Fmoc-Thr(tBu)-alcohol (Fmoc-Thr(tBu)-ol) Fmoc-Thr(tBu)-OH (1 eq, 0.663 g, 1.67 mmol) was suspended in 2 ml of ethylene glycol dimethyl ether (DME) and chilled below −15° C. under nitrogen. After addition of N-methylmorpholine (1 eq, 0.19 ml, 1.67 mmol) and isobutyl chloroformate (1 eq, 0.22 ml, 1.67 mmol), the mixture was stirred at −15° C. After 5 min of stirring, the precipitate was removed, and a suspension of NaBH4 (3 eq) in 5 ml of water was added and stirred for another 1 hr. At the end of the reaction, 40 ml of water was added. The mixture was extracted with DCM (20 ml×3), and the combined organic layers were washed with 5% NaHCO3, followed by rinsing with brine (e.g., NaCl), and being dried over anhydrous Na2SO4 (or MgSO4). The solvent was evaporated. Crude Fmoc-Thr (tBu)-alcohol was purified by silica gel column chromatography using DCM as an eluent: 1H-NMR (CDCl3) δ (Ppm): 1.16 (3H, d, J=6.2 Hz, CHCH3), 1.20 (9H, s, tBu), ...
example 2
Preparation of Peptidyl-Resins
[0193] The peptidyl-resins were prepared according to the Merrified solid phase synthesis techniques (See Steward and Young, Solid Phase Peptide Synthesis, 2nd edition, Pierce Chemical Company, Rockford, (1984) and Merrified, J. Am. Chem. Soc. 85, 2149-2154 (1963)). In the present invention, a Wang resin, a 2-chlorotrityl chloride resin, and a Rank amide resin in the Fmoc synthetic techniques were used. The Wang resin was used to synthesize the peptidyl moieties in which they have carboxylic acid moiety at a C-terminus. A 2-Chlorotrityl chloride resin was used to synthesize the peptidyl moieties in which they have Pro, Cys, or amino alcohols at a C-terminus. A Rink amide resin was used to synthesize the peptidyl moieties in which they have amide at a C-terminus. Applications of these resins in SPPS were described in the art, for examples, S.-S. Wang, J. Am. Chem. Soc., 95, 1328 (1973) and G. Lu, et al., J. Org. Chem., 46, 3433 (1981) for the Wang resin...
example 3
Synthesis of End-Group Functionalized PEG Derivatives
[0196] Carboxyl-PEG and its Active Esters
[0197] Carboxyl-PEG. PEG2000 (8.6 g) and potassium tert-butoxide (20 g) were dissolved in 300 ml tert-butyl alcohol and warmed to 40° C. Ethyl bromoacetate (10 ml) was added over a period of 20 min. The mixture was stirred for 2 hr and then evaporated to remove solvent. The residue was hydrolyzed in 200 ml of 1 N NaOH and stirred at room temperature for 2 hrs. At the end of hydrolysis, the pH of the mixture was adjusted to 2 and extracted by CHCl3 (2×200 ml). The combined extract was washed with water, dried over anhydrous MgSO4, evaporated to concentrate and dried in a vacuum. A white Carboxyl-PEG powder was obtained and yielded 6.88 g. 1H-NMR (CDCl3) δ (ppm): 3.66 (s, O—CH2CH2—O), 4.13 (s, HO—C(O)—CH2—O).
[0198] PEG-oxybenzotriazole HOBt (2.6 mmol), DIPCDI (1.91 mmol), and carboxyl-PEG3000 (0.87 mmol) were mixed in 4 ml DMF and stirred at room temperature under nitrogen for 20 min. The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com