System and method for monitoring health using exhaled breath
a technology of exhalation and health monitoring, which is applied in the field of non-invasive monitoring of substance/compound concentrations in blood, can solve the problems of ineffective medication, high incidence of side effects of tcas, and toxic to the body of certain medications, and achieve the effect of cost-effectiveness and frequentity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Intravenous IV Anesthesia Delivery
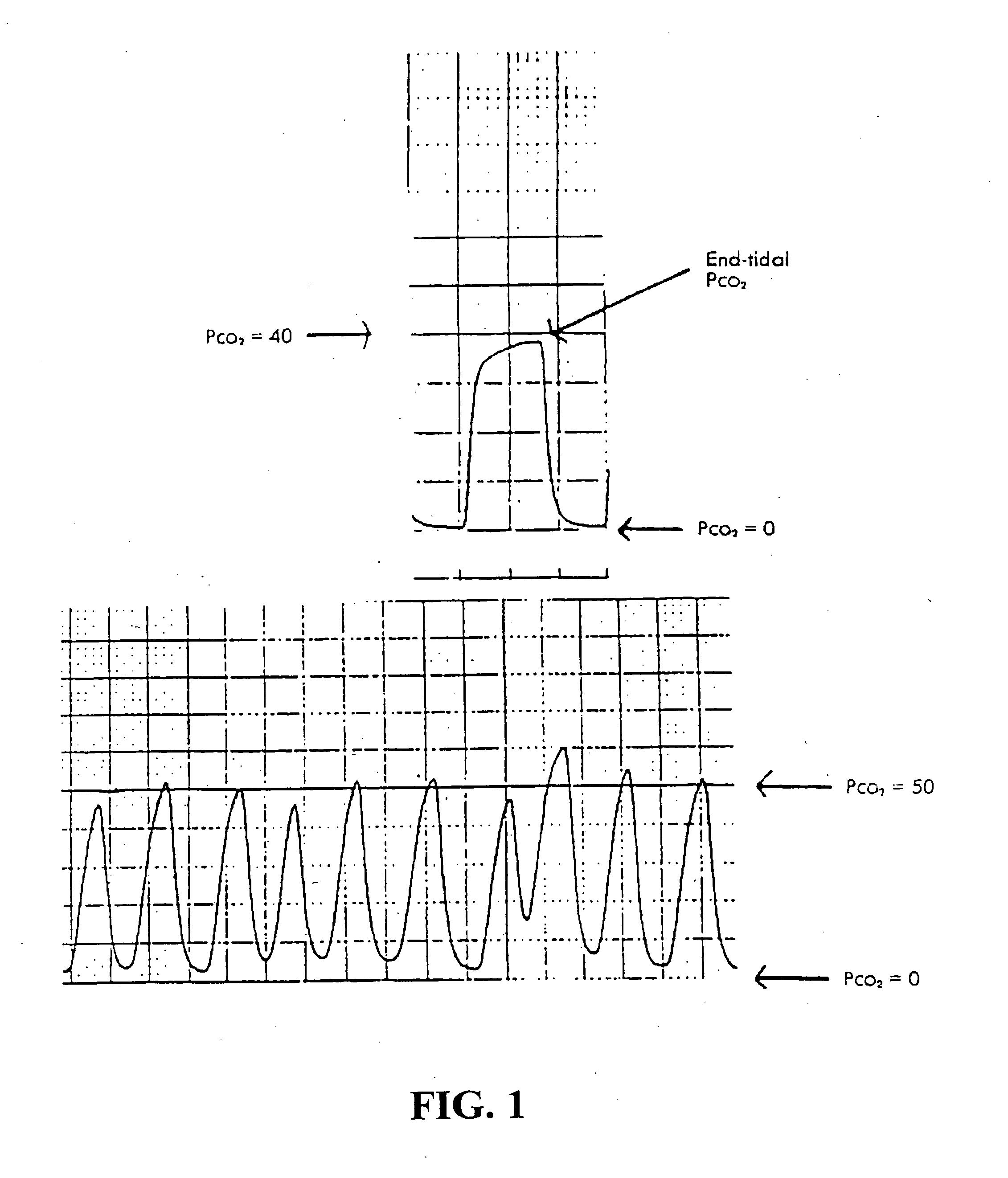
[0179] During intravenous anesthesia, anesthetic agents are administered directly into a patient's bloodstream rather than administering gases through a breathing circuit. The administered drug may bind to proteins circulating in the blood, be absorbed into fat or exist in a “free” form. Drug bound to protein or absorbed in fat does not produce a pharmacological effect and exists in equilibrium with unbound drug. Numerous factors, including competition for binding sites on the protein from other drugs, the amount of fat in the body and the amount of protein produced, determine the equilibrium between bound and unbound drug. Unbound drug may participate directly in the pharmacological effect or be metabolized into a drug that produces the effect. Metabolism of the active drug often leads to its removal from the bloodstream and termination of its effect. The drug effect can also be terminated by the excretion of the free drug. Free drug or a metaboli...
example 2
Inhalational Anesthesia
[0191] Inhalation agents are generally administered through a breathing system. A breathing system is an assembly of components which connects the patient's airway to the anesthetic machine, from and into which the patient breathes. As known in the art, such systems generally include a fresh gas entry port / delivery tube through which the gases are delivered from the machine; a port to connect it to the patient's airway (oral airway, mask, endotracheal tube); a reservoir for gas; a expiratory port / valve through which the expired gas is vented to the atmosphere; a carbon dioxide absorber (for rebreathing); and tubes for connecting these components. Flow directing valves may or may not be used.
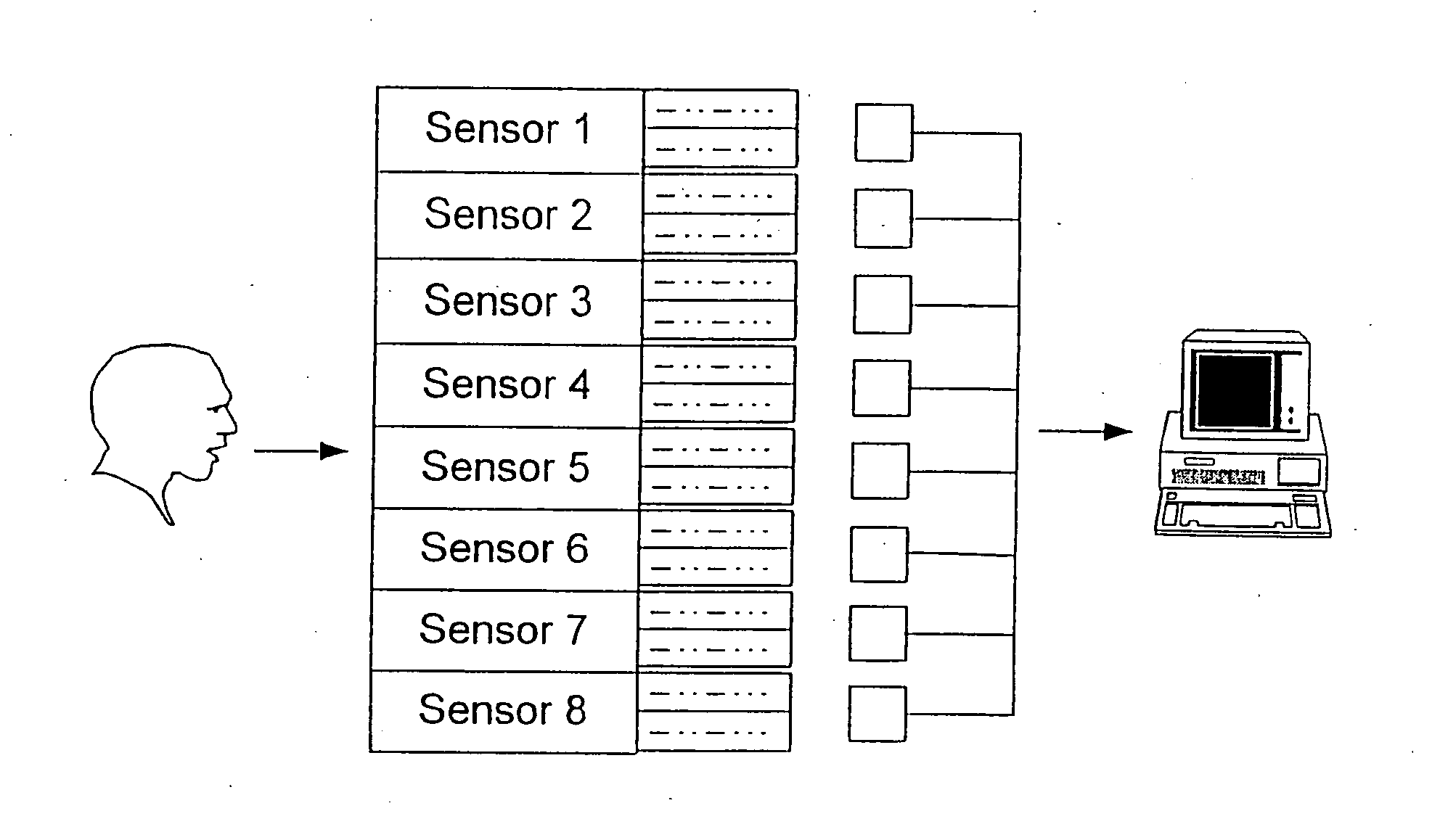
[0192] The sensors of the present invention are in communication with the delivered (inspired) gas and / or the expired gas of the breathing circuit to appropriately monitor the target substance(s). Preferably, the sensors are in flow communication with the appropriate tube...
example 3
Selection of Sensors
[0196] The following are examples of various sensor technologies that may be utilized in practicing the method of the present invention:
Microgravimetric Sensors
[0197] Microgravimetric sensors are based on the preparation of polymeric- or biomolecule-based sorbents that are selectively predetermined for a particular substance, or group of structural analogs. A direct measurement of mass changes induced by binding of a sorbent with a target marker can be observed by the propagation of acoustic shear waves in the substrate of the sensor. Phase and velocity of the acoustic wave are influenced by the specific adsorption of target markers onto the sensor surface. Piezoelectric materials, such as quartz (SiO2) or zinc oxide (ZnO), resonate mechanically at a specific ultrasonic frequency when excited in an oscillating field. Electromagnetic energy is converted into acoustic energy, whereby piezoelectricity is associated with the electrical polarization of materials w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com