Apparatus for electrolysis of molten oxides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
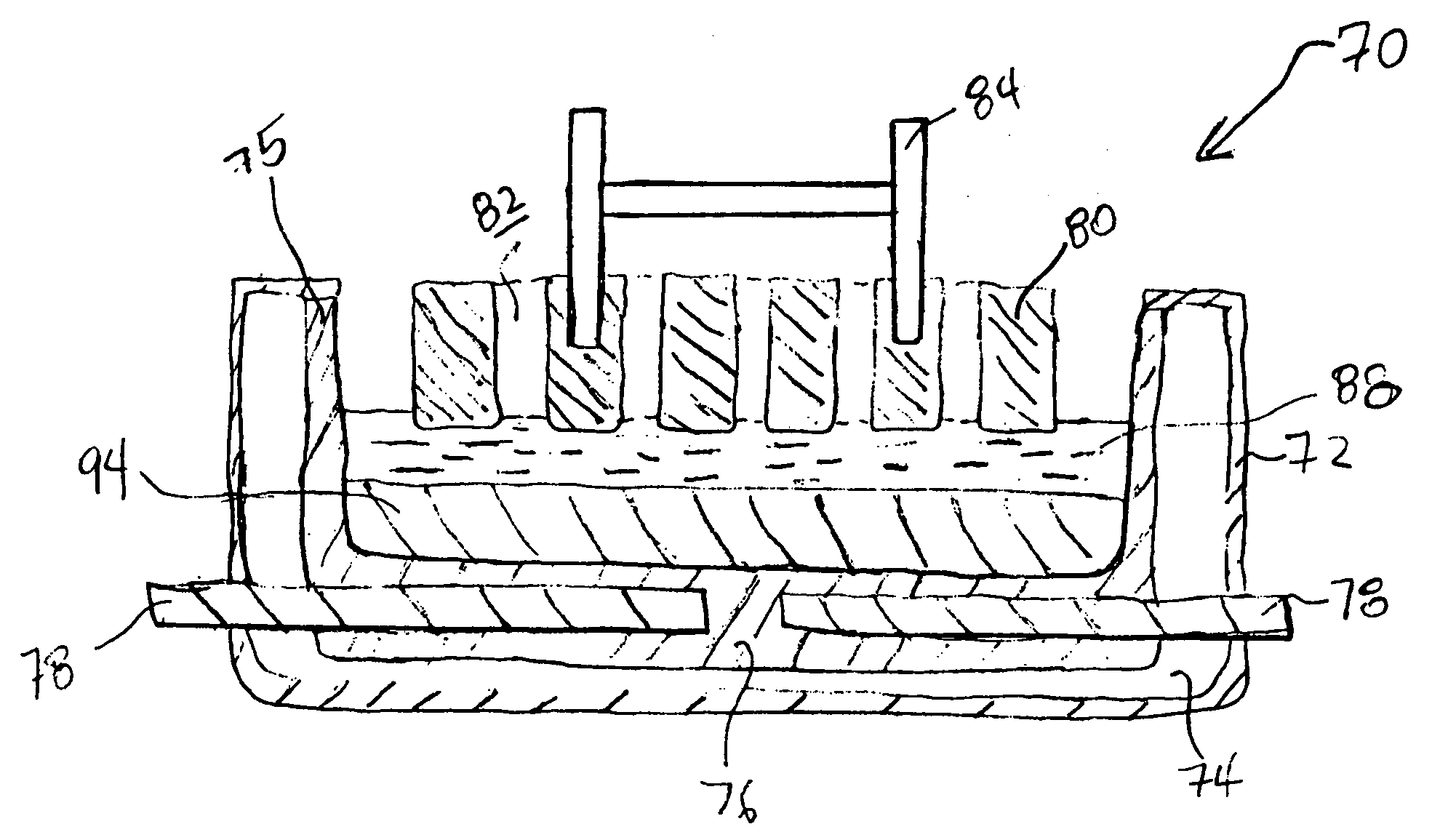
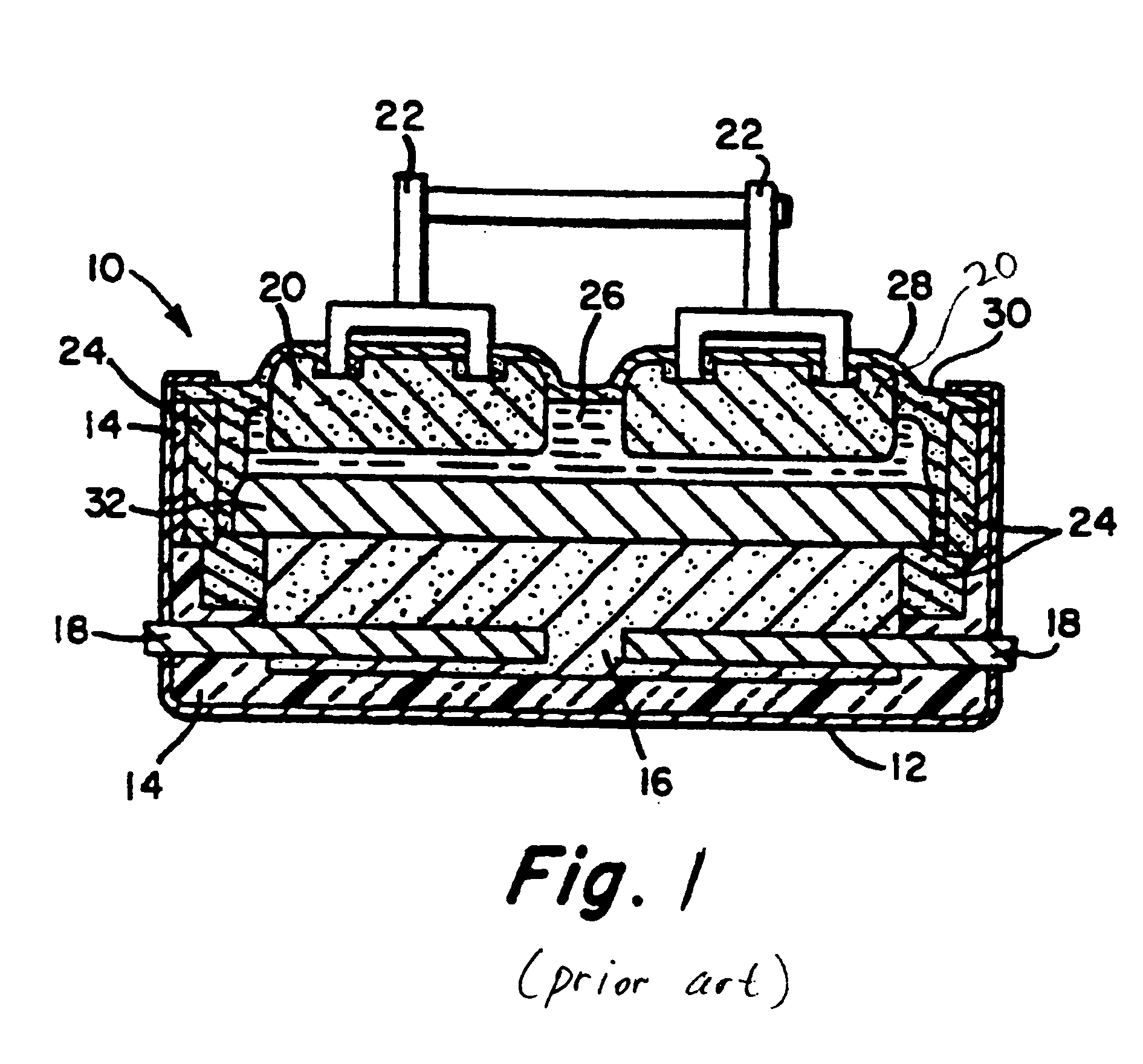
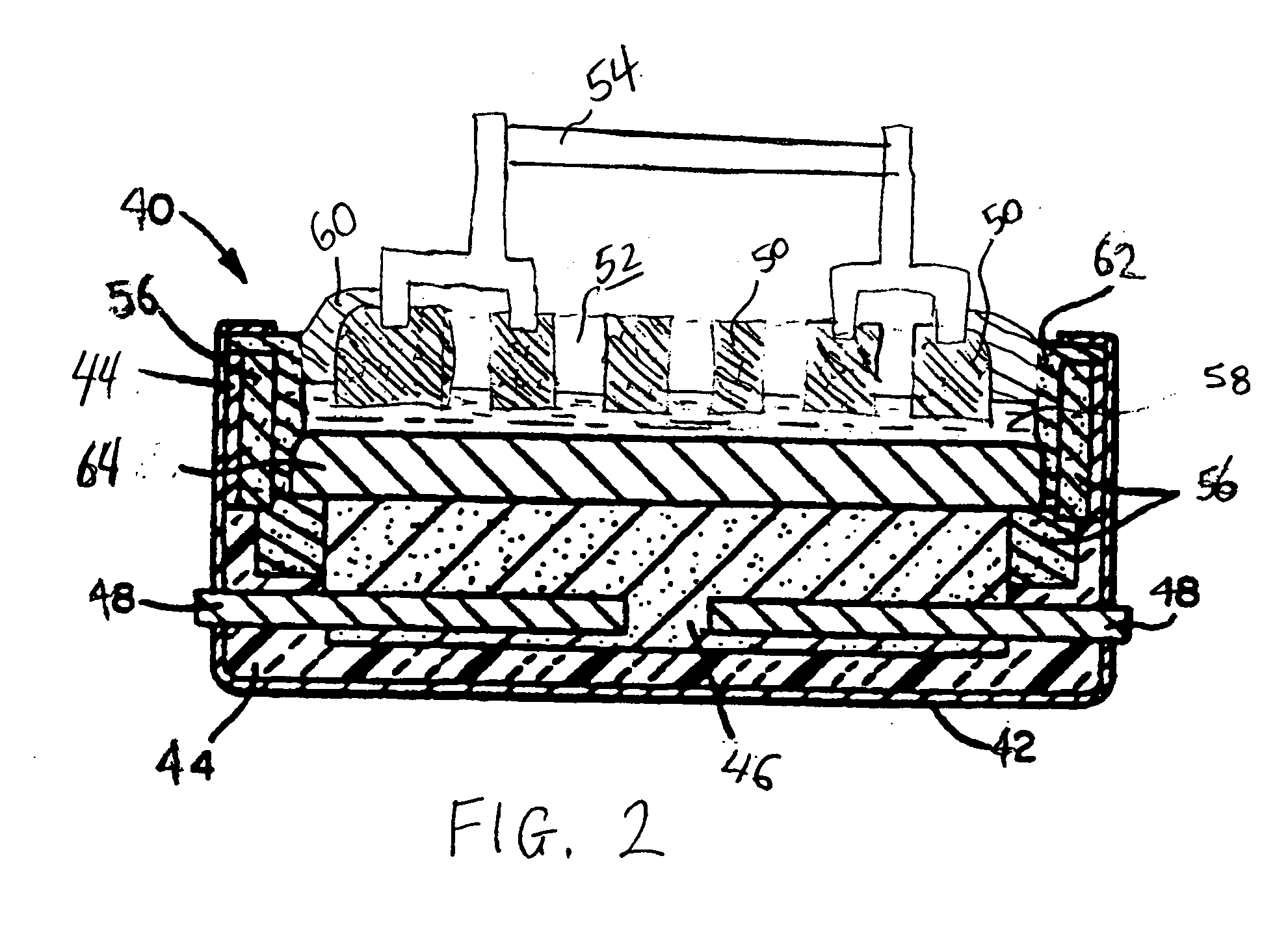
[0047]With reference to FIG. 1, a conventional Hall-Héroult cell 10, of the prior art, has a steel outer shell 12 lined by overlying thermal insulation 14. A carbon cathode substrate 16 positioned at the bottom of the cell 10 contains metallic current collector bars 18. Carbon anodes 20 are formed by prebaking carbon blocks suspended from steel anode rods 22 which supply electrical current to the anodes 20. A cell lining 24 is also formed from carbon blocks.
[0048]Molten electrolyte 26 contains dissolved alumina, which is continually supplied by breaking an alumina crust 28 and adding fresh alumina. The alumina crust 28 forms on frozen electrolyte and helps to minimize heat loss from the top of the cell 10. Since cryolite, Na3AlF6, has the capacity to dissolve alumina, it is the principal constituent of the electrolyte 26. Additionally, certain fluoride salts are present in the electrolyte 26. Calcium fluoride, CaF2, decreases the freezing point of cryolite. Aluminum fluoride, AlF3 c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com