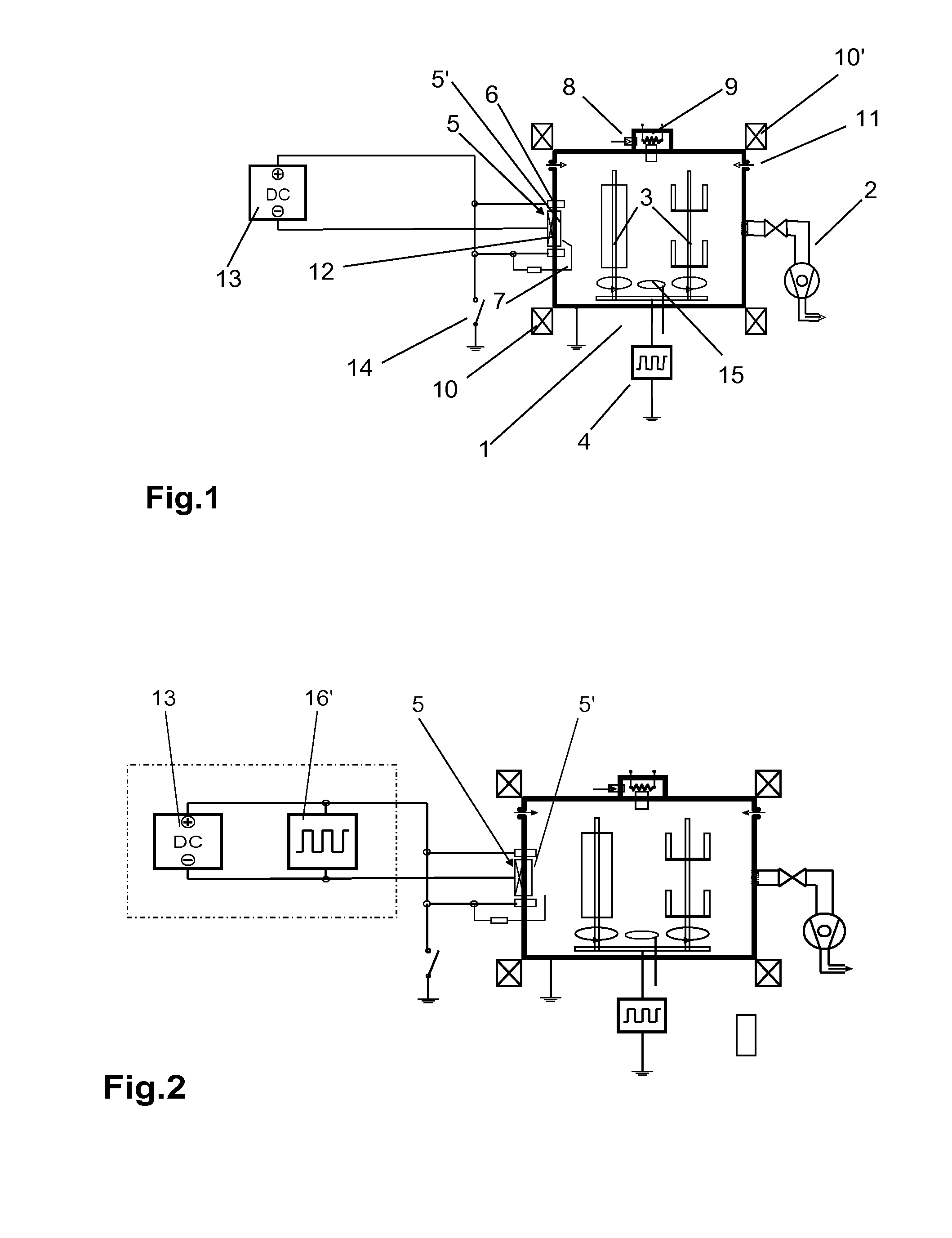
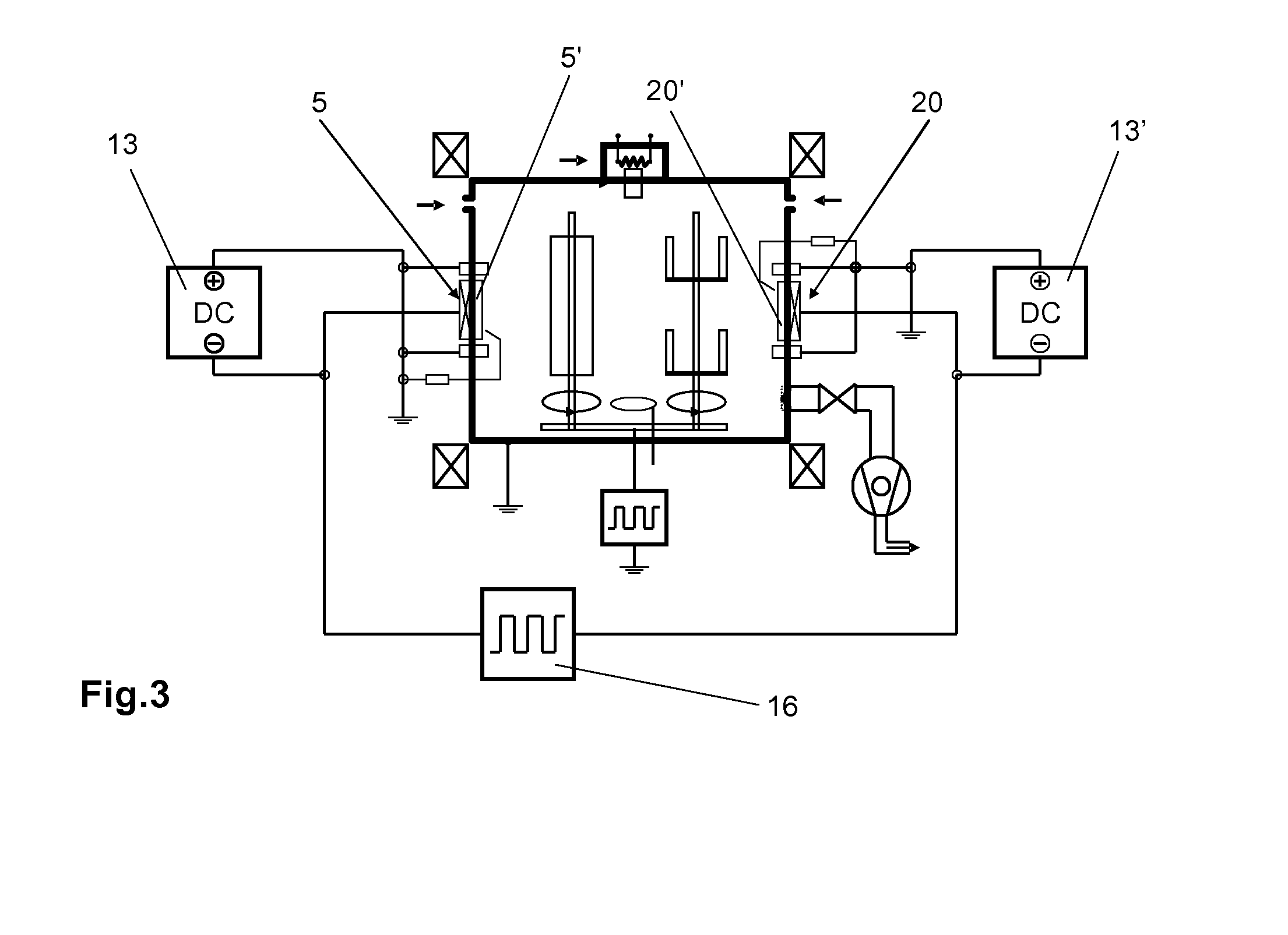
A significant
disadvantage with these sources comprises that in the proximity of the
cathode spot very rapidly proceeding melting occurs on the
target surface, whereby drops are formed, so-called droplets, which are hurled away as splatters and subsequently condense on the workpiece and consequently have an undesirable effect on the layer properties.
With high requirements made of the layer quality,
layers generated thusly, can often not be commercially applied.
In the production of electrically nonconducting, thus
dielectric layers, such as for example of oxides using
oxygen as the
reactive gas, the problem of splatter formation is intensified.
The re-
coating of the target surfaces of the arc
evaporator and of the counterelectrodes, such as the anodes and also other parts of the vacuum process installation, with a non-conducting layer leads to entirely unstable conditions and even to the
quenching of the arc.
In this case the latter would have to be repeatedly newly ignited or it would thereby become entirely impossible to conduct the process.
In the reactive coating by means of arc evaporator source there is a lack of reactivity and process stability, especially in the production of insulating layers.
Working with
high frequency, such as is the case during
sputtering, has so far failed due to the lacking technique of being able to operate high-power supplies with high frequencies.
In oxidized target surfaces a renewed igniting with mechanical contact and by means of DC supplies is not possible.
The actual problem in reactive arc
evaporation are the coatings with insulating layers on the target and the
anode, or on the coating chamber connected as the
anode.
In the course of their formation, these insulating coatings increase the burn
voltage of the
spark discharge, lead to increased splatters and sparkovers, an unstable process, which ends in an interruption of the
spark discharge.
While the proposal according to U.S. Pat. No. 5,103,766 of alternately operating the
cathode and the
anode with renewed ignition each time results in process stability, it does however lead to increased splatters.
The method most frequently mentioned is dual magnetron
sputtering, which in this application entails great disadvantages with respect to process reliability and costs.
However, this does not necessarily make the process less expensive and faster.
Until now it also did not appear possible to be able to produce satisfactorily alpha aluminum
oxide layers by means of arc
evaporation.
With respect to prior art the following disadvantages are summarized, in particular regarding the production of oxidic layers with reactive process:1. Stable conduction of the process is not possible for the deposition of insulating layers, if there is no spatial separation between arc evaporator
cathode or anode of the arc
discharge and the substrate region with
reactive gas inlet.
2. There is no
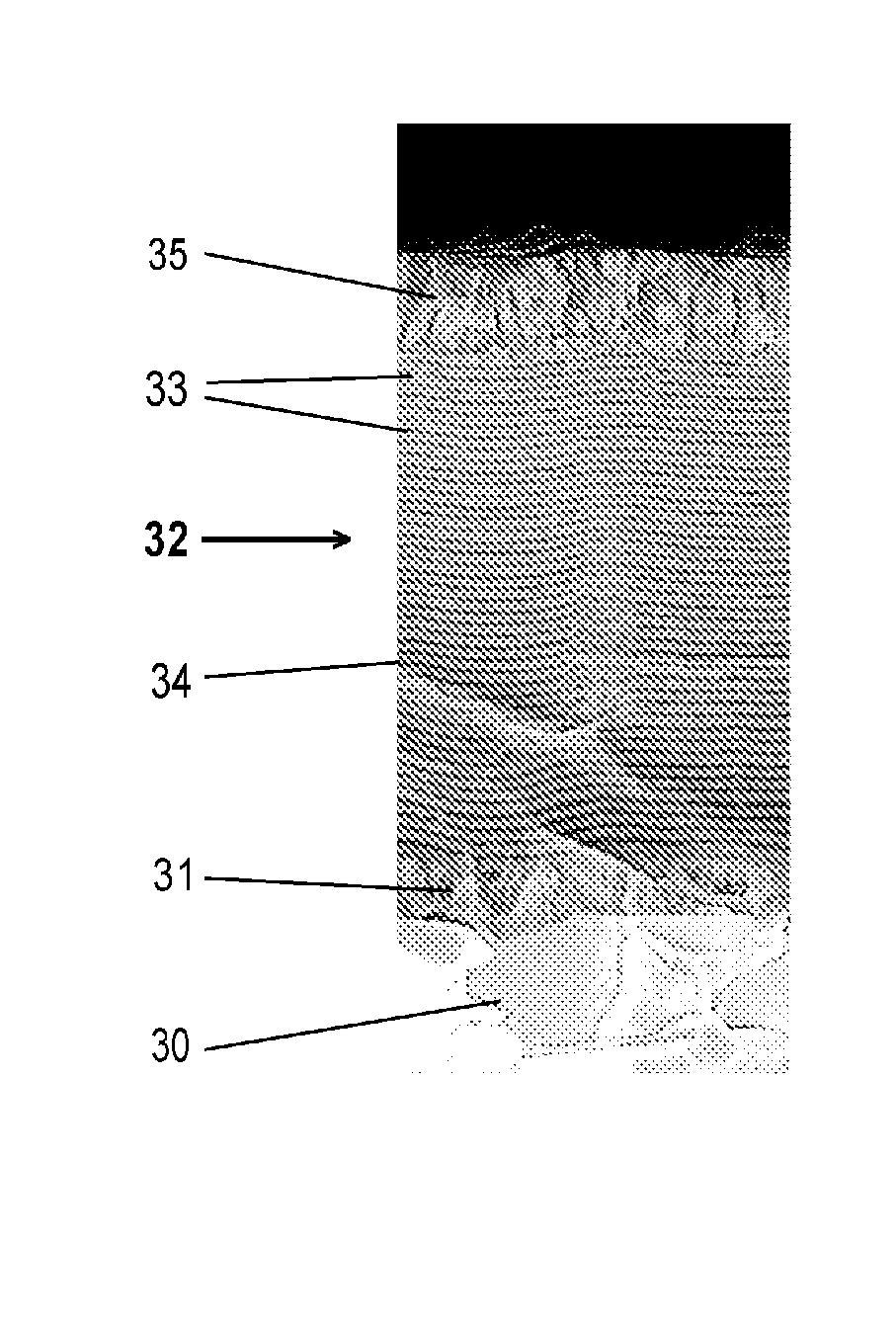
fundamental solution of the problematic of splatters: conglomerates (splatters) are not fully through-reacted, wherein metallic components occur in the layer, increased roughness of the layer surface is generated and the constancy of the layer composition and the
stoichiometry is disturbed.3. Insufficient possibilities for realizing low-temperature processes, since insufficiently the thermal loading of the substrate is too high for the production of oxides with high-temperature phases.4. The production of flat graduated intermediate layers for insulating layers has so far not been possible by means of arc evaporation.
These conglomerates impinge as such onto the substrate and result in rough layers, and it has not been possible fully to react-through the splatters.
Avoidance or fragmentation of these splatters was so far not successful, especially not for reactive coating processes.
In these, on the cathode of the arc evaporator source, for example in
oxygen atmosphere, additionally a
thin oxide layer forms, which tends to increased splatter formation.
 Login to View More
Login to View More