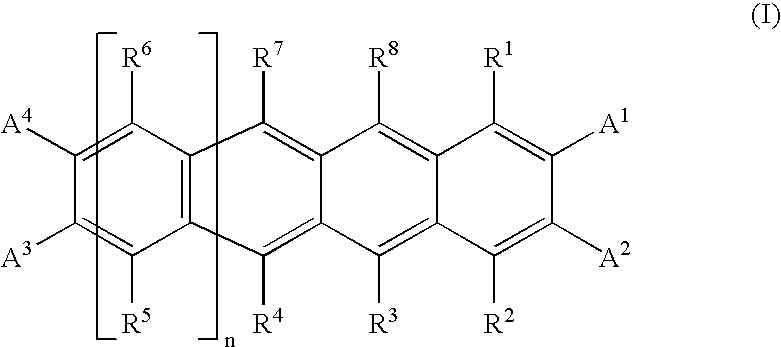
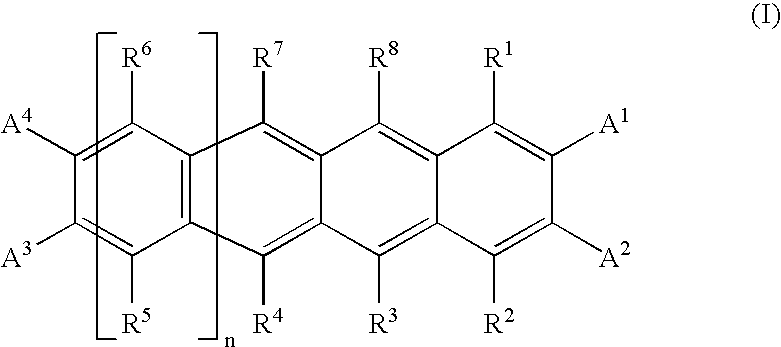
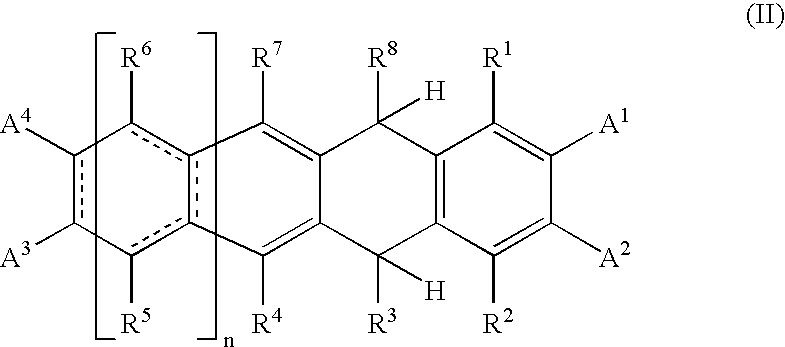
Photoelectric Conversion Devices
a technology of photoelectric conversion and material, applied in the field of material for photoelectric conversion devices, can solve the problems of large quantity of energy required to produce the device, inability to consider the effectiveness of solar cells using inorganic semiconductors at the present stage, and influence on the existence of humans, etc., to achieve low toxicity, easy to adapt, and good workability and productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
Synthesis of Compound 1 of the Present Invention
[0098]Compound 1 of the present invention represented by the following formula was synthesized as follows.
[0099]To zirconocene dichloride dissolved in THF, 2 equivalents of n-butyllithium were added at −78° C., and the resultant mixture was stirred for 1 hr. To this solution was added 1 equivalent of Diyne 1, the compound shown below, and the mixture was stirred at room temperature to generate Zirconacyclopentadiene 1, the compound shown below. To this compound were added 2 equivalents of NiCl2(PPh3)2 complex and 1 equivalent of diphenylacetylene, and the resultant mixture was stirred at 50° C. for 3 hr to obtain dihydro-compound of Compound 1, which was reacted with 1 equivalent of 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) to obtain Compound 1 as red crystals. Compound 1 obtained here was purified by sublimation and used for evaluation of the photoelectric conversion device described below.
synthesis example 2
Synthesis of Compound 2 of the Present Invention
[0100]Compound 2 of the present invention represented by the following formula was synthesized as follows.
[0101]To zirconocene dichloride dissolved in THF, 2 equivalents of n-butyllithium were added at −78° C., and the resultant mixture was stirred for 1 hr. To this solution was added 1 equivalent of Diyne 2, the compound shown below, and the mixture was stirred at room temperature to generate Zirconacyclopentadiene 2, the compound below. To this compound were added 2 equivalents of CuCl, 3 equivalents of N,N-dimethylpropyleneurea (DMPU), and 1 equivalent of dimethyl acetylenedicarboxylate (DMAD), and the resultant mixture was stirred at 50° C. for 3 hr to obtain dihydro-compound of Compound 2, which was reacted with 1 equivalent of 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) to obtain Compound 2. Compound 2 obtained here was purified by sublimation and used for evaluation of the photoelectric conversion device described below.
synthesis example 3
Synthesis of Compound 3 of the Present Invention
[0102]Compound 3 of the present invention represented by the following formula was synthesized as follows.
[0103]To zirconocene dichloride dissolved in THF, 2 equivalents of n-butyllithium were added at −78° C., and the resultant mixture was stirred for 1 hr. To this solution was added 0.5 equivalent of Tetrayne 3, the compound shown below, and the mixture was stirred at room temperature to generate Zirconacyclopentadiene 3, the compound below. To this compound were added 4 equivalents of NiCl2(PPh3)2 complex and 2 equivalents of 5-decyne, and the resultant mixture was stirred at 50° C. for 3 hr to obtain tetrahydro-compound of Compound 3, which was reacted with 2 equivalents of chloranil to obtain Compound 3. Compound 3 obtained here was purified by sublimation and used for evaluation of the photoelectric conversion device described below.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com