Treatment of cellulosic material and enzymes useful thererin
a cellulosic material and enzyme technology, applied in the field of cellulosic material production of sugar hydrolysates, can solve the problems of high bioethanol production cost, low energy output, and insatiable technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Screening for Strains Expressing Cellulolytic Activity and their Cultivation for Purification
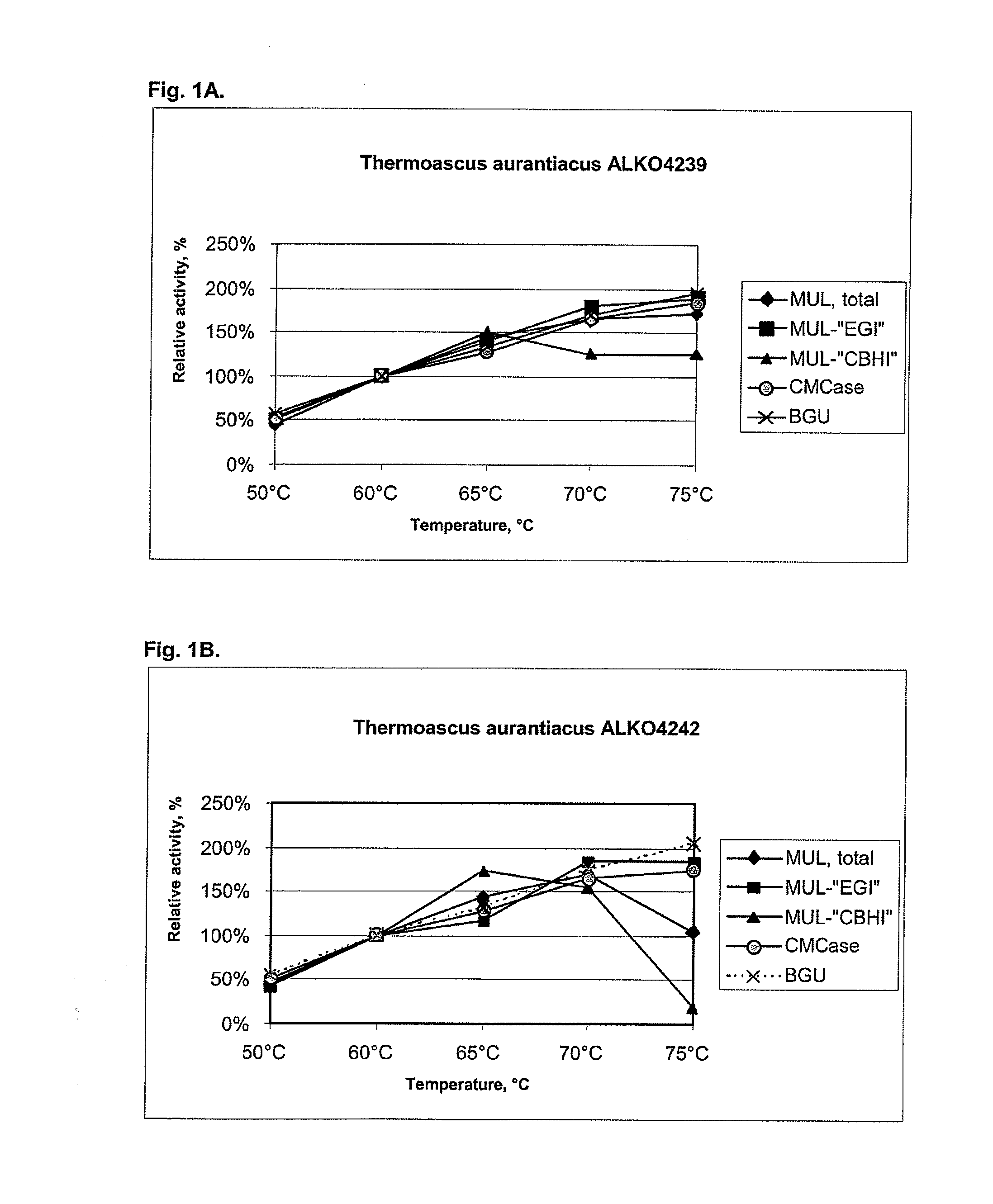
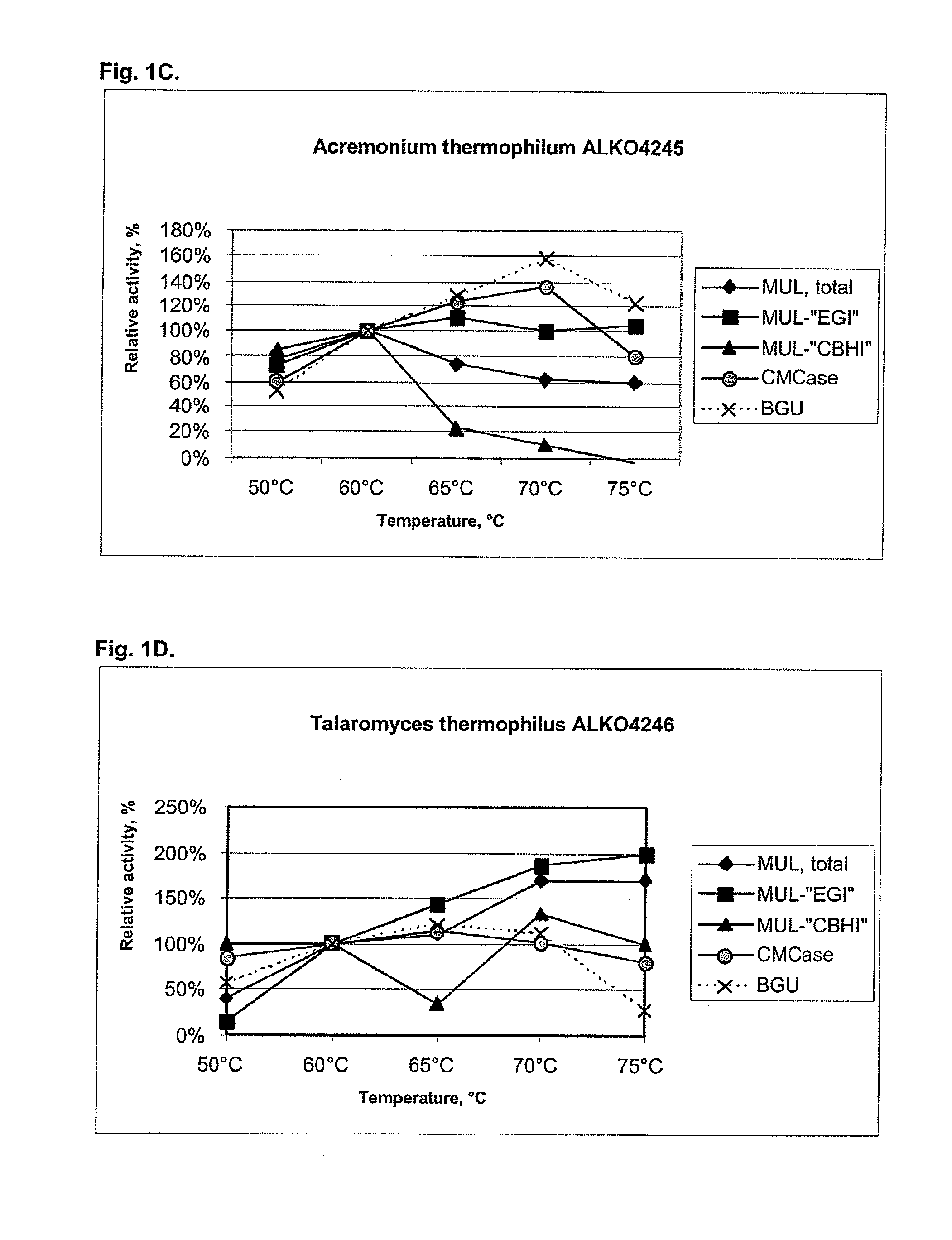
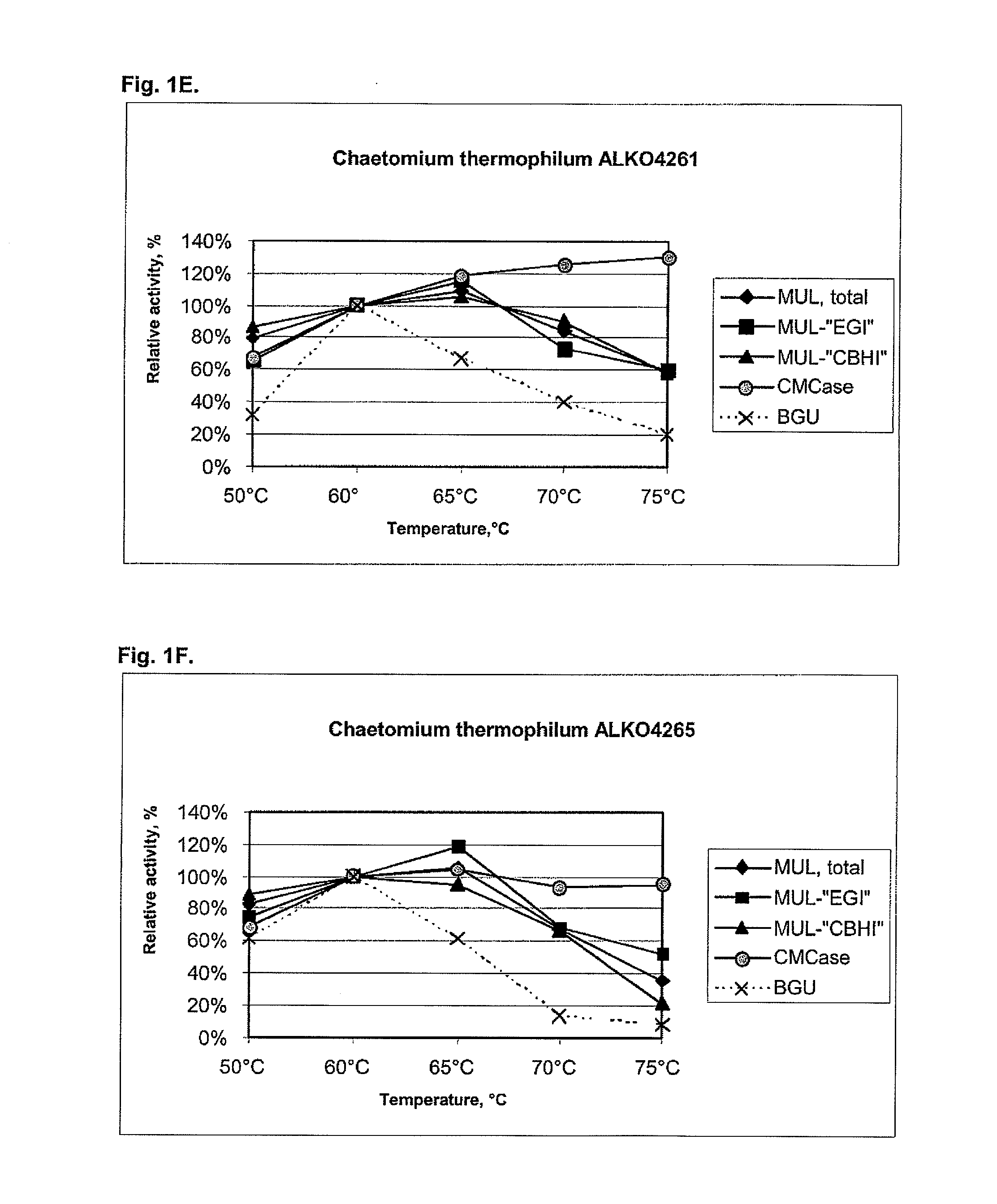
[0104]About 25 fungal strains from the Roal Oy culture collection were tested for cellulolytic activity including beta-glucosidases. After preliminary screening six strains were chosen for further studies. These were Thermoascus aurantiacus ALKO4239 and ALKO4242, Acremonium thermophilum ALKO4245, Talaromyces thermophilus ALKO4246 and Chaetomium thermophilum ALKO4261 and ALKO4265.
[0105]The strains ALKO4239, ALKO4242 and ALKO4246 were cultivated in shake flasks at 42° C. for 7 d in the medium 3×B, which contains g / litre: Solka Floc cellulose 18, distiller's spent grain 18, oats spelt xylan 9, CaCO3 2, soybean meal 4.5, (NH4)HPO4 4.5, wheat bran 3.0, KH2PO4 1.5, MgSO4.H2O 1.5, NaCl 0.5, KNO3 0.9, locust bean gum 9.0, trace element solution #1 0.5, trace element solution #2 0.5 and Struktol (Stow, Ohio, USA) antifoam 0.5 ml; the pH was adjusted to 6.5. Trace element solution #1 has g / litre: MnSO...
example 2
Purification and Characterization of Cellobiohydrolases from Acremonium thermophilum ALKO4245 and Chaetomium thermophilum ALKO4265
[0122]Acremonium thermophilum ALKO4245 and Chaetomium thermophilum ALKO4265 were grown as described in Example 1. The main cellobiohydrolases were purified using p-aminobenzyl 1-thio-β-cellobioside-based affinity column, prepared as described by Tomme et al., 1988.
[0123]The culture supernatants were first buffered into 50 mM sodium acetate buffer pH 5.0, containing 1 mM δ-gluconolactone and 0.1 M glucose in order to retard ligand hydrolysis in the presence of β-glucosidases. Cellobiohydrolases were eluted with 0.1 M lactose and finally purified by gel filtration chromatography using Superdex 200 HR 10 / 30 columns in the ÄKTA system (Amersham Pharmacia Biotech). The buffer used in gel filtration was 50 mM sodium phosphate pH 7.0, containing 0.15 M sodium chloride.
[0124]Purified cellobiohydrolases were analysed by SDS-polyacrylamide gel electrophoresis and t...
example 3
Purification and Characterization of an Endoglucanase from Acremonium thermophilum ALKO4245
[0128]Acremonium thermophilum ALKO4245 was grown as described in Example 1. The culture supernatant was incubated at 70° C. for 24 hours after which it was concentrated by ultrafiltration. The pure endoglucanase was obtained by sequential purification with hydrophobic interaction and cation exchange chromatography followed by gel filtration. The endoglucanase activity of the fractions collected during purification was determined using carboxymethyl cellulose (CMC) as substrate (procedure of IUPAC 1987). Protein content was measured by BioRad Assay Kit (Bio-Rad Laboratories) using bovine serum albumine as standard.
[0129]The concentrated culture supernatant was applied to a HiPrep 16 / 10 Butyl FF hydrophobic interaction column equilibrated with 20 mM potassium phosphate buffer pH 6.0, containing 1 M (NH4)2SO4. Bound proteins were eluted with the linear gradient from the above buffer to 5 mM potas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com