Abuse-resistant amphetamine prodrugs
a technology of amphetamine and prodrugs, applied in the field of amphetamine compounds, can solve the problems of temporary exhilaration, amphetamine abuse, amphetamine derivatives and analogs,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Synthesis of Peptide Amphetamine Conjugates
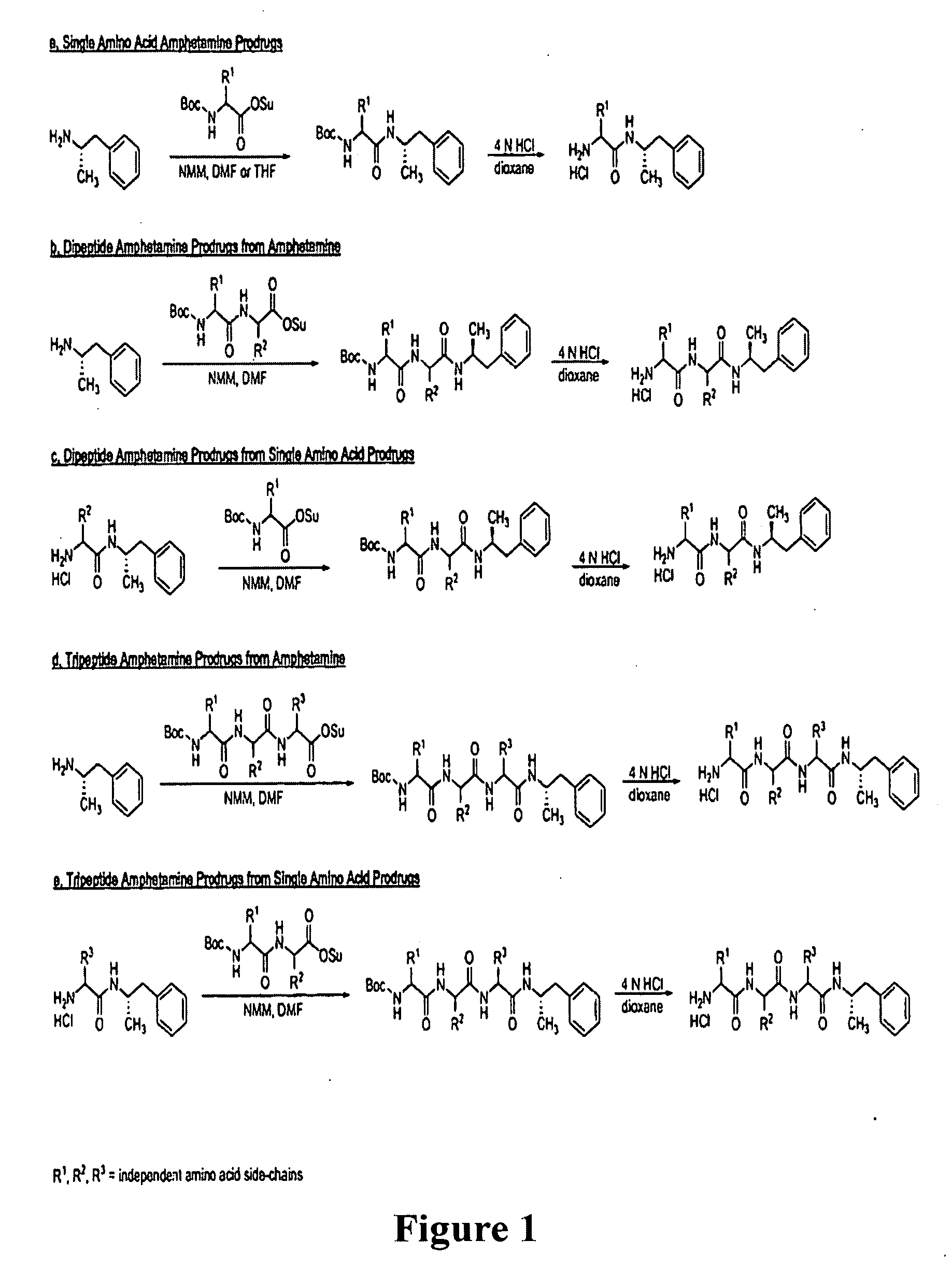
[0172]Peptide conjugates were synthesized by the general method described in FIG. 1. An iterative approach can be used to identify favorable conjugates by synthesizing and testing single amino acid conjugates, and then extending the peptide one amino acid at a time to yield dipeptide and tripeptide conjugates, etc. The parent single amino acid prodrug candidate may exhibit more or less desirable characteristics than its di- or tripeptide offspring candidates. The iterative approach can quickly suggest whether peptide length influences bioavailability.
General Synthesis of Single Amino Acid Amphetamine Conjugates
[0173]To a solution of a protected amino acid succinimidyl ester (2.0 eq) in 1,4-dioxane (30 mL) was added d-amphetamine sulfate (1.0 eq) and NMM (4.0 eq). The resulting mixture was allowed to stir for 20 h at 20° C. Water (10 mL) was added, and the solution was stirred for 10 minutes prior to removing solvents under reduced p...
example 2
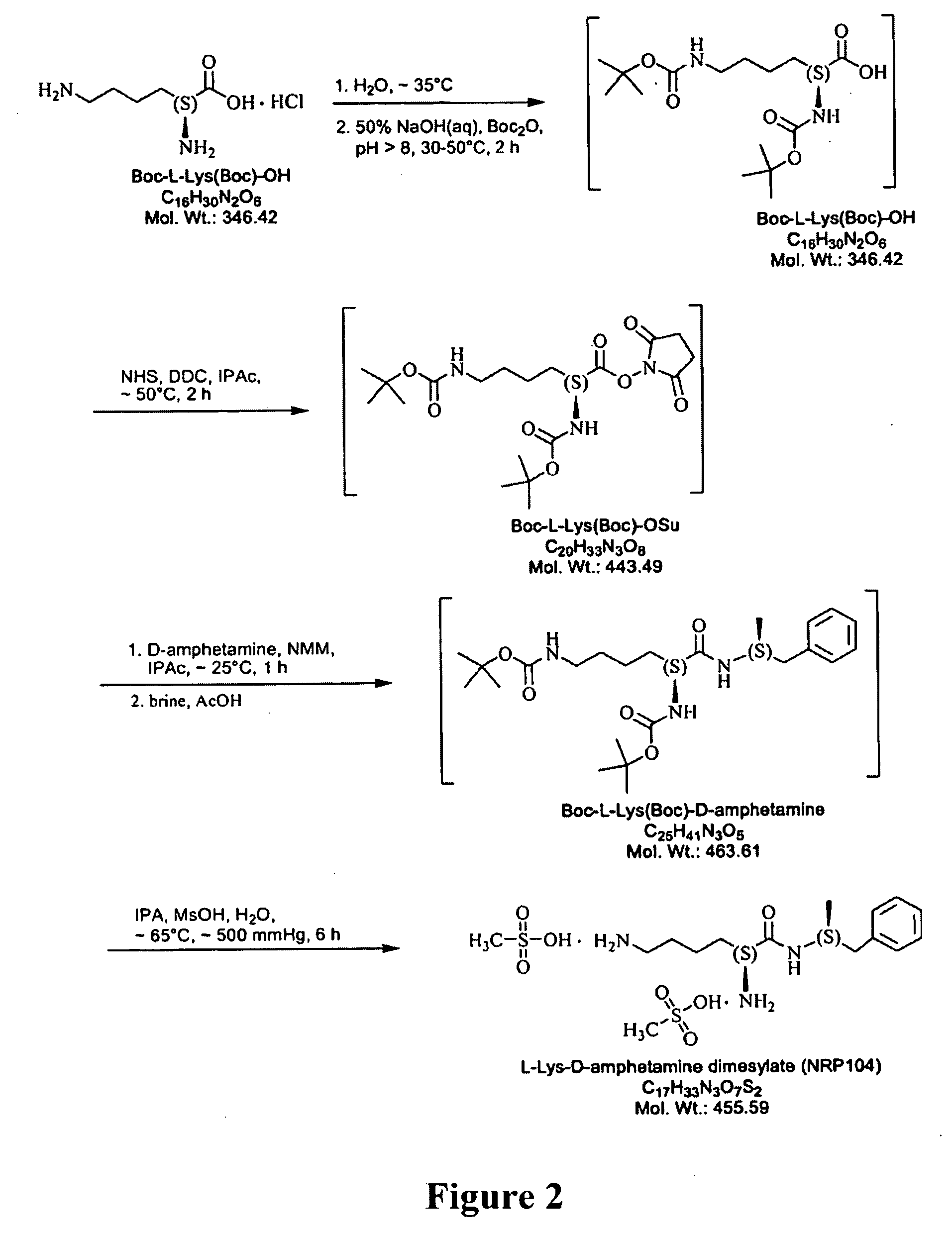
Synthesis of L-lysine-d-amphetamine
[0177]L-lysine-d-amphetamine was synthesized by the following methods.
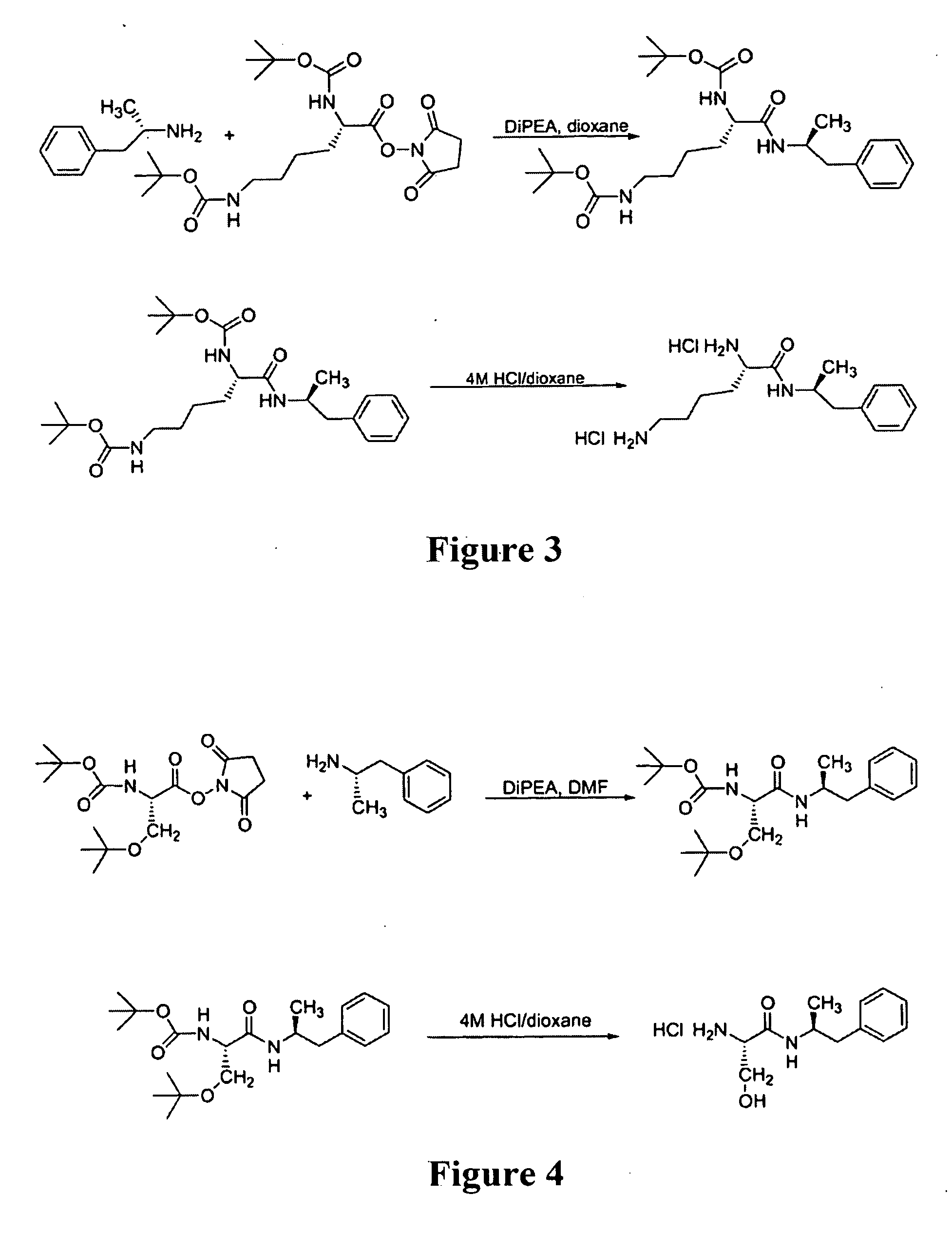
[0178]a. Preparation of HCl salt (see FIG. 3)
[0179]i. Coupling
ReagentsMWWeightmmolesMolar Equivalentsd-amphetamine135.24.75g35.131free baseBoc-Lys(Boc)-OSu443.515.58g35.131Di-iPr-Et-Amine129906mg 7.030.2, d = 0.74, 1.22 mL1,4-Dioxane—100mL——
[0180]To a solution of Boc-Lys(Boc)-OSu (15.58 g, 35.13 mmol) in dioxane (100 mL) under an inert atmosphere was added d-amphetamine free base (4.75 g, 35.13 mmol) and DIPEA (0.9 g, 1.22 mL, 7.03 mmol). The resulting mixture was allowed to stir at room temperature overnight. Solvent and excess base were then removed using reduced pressure evaporation. The crude product was dissolved in ethyl acetate and loaded on to a flash column (7 cm wide, filled to 24 cm with silica) and eluted with ethyl acetate. The product was isolated, the solvent reduced by rotary evaporation, and the purified protected amide was dried by high-vac to obtain a white sol...
example 3
Synthesis of Ser-Amp
[0192]Ser-Amp was synthesized by a similar method (see FIG. 4) except the amino acid starting material was Boc-Ser(O-tBu)-OSu and the deprotection was done using a solution of trifluoroacetic acid instead of HCl.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com