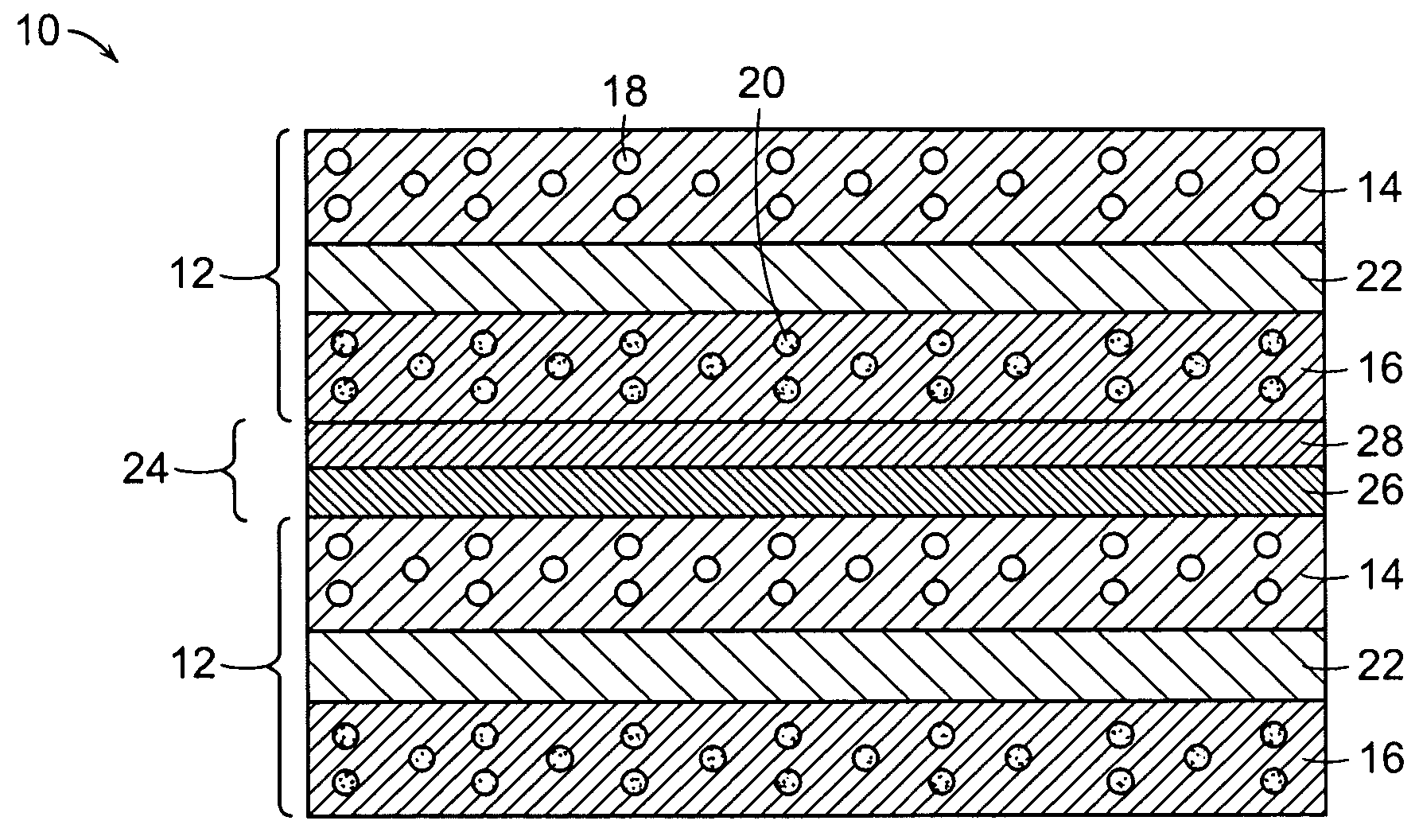
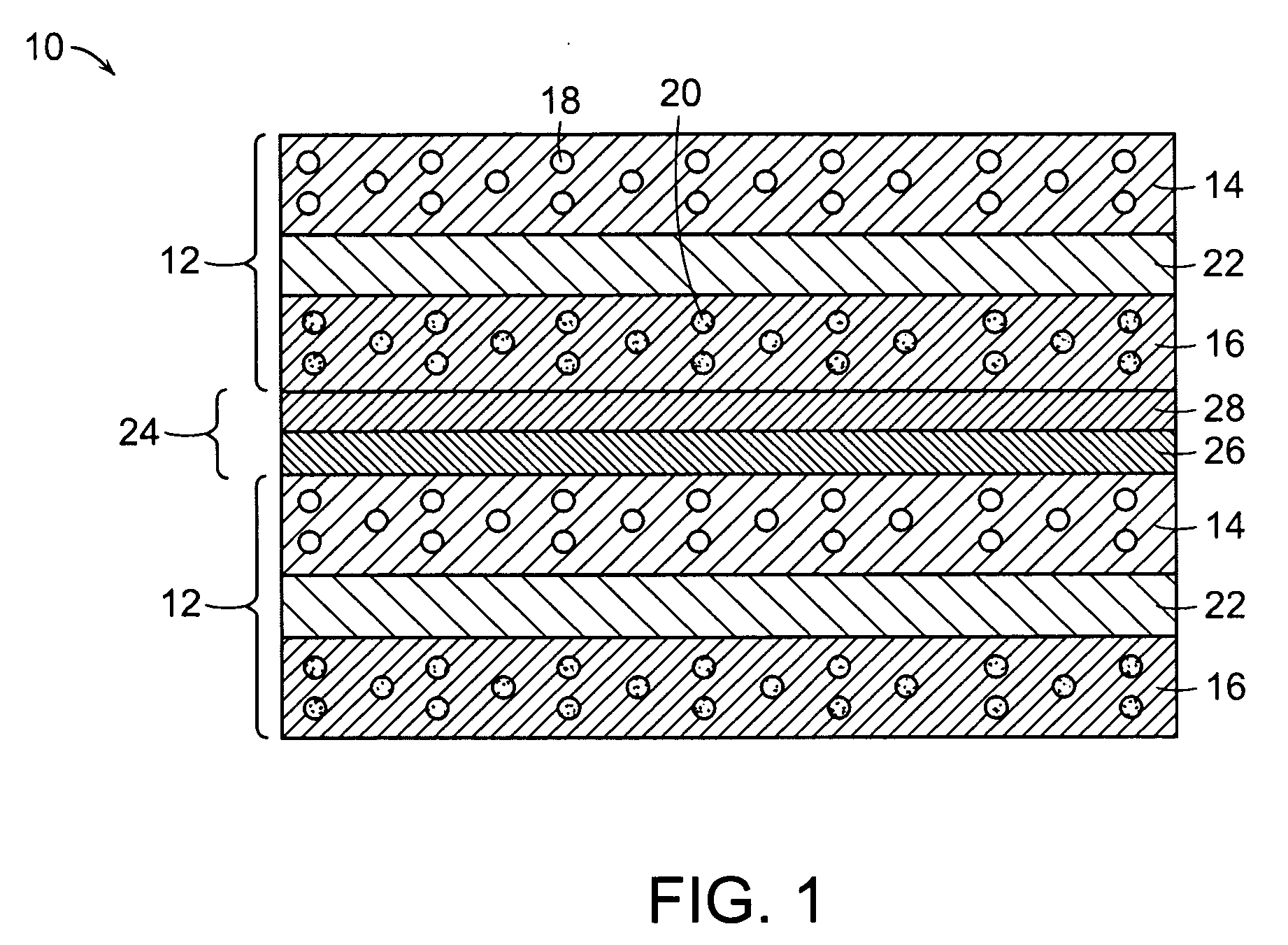
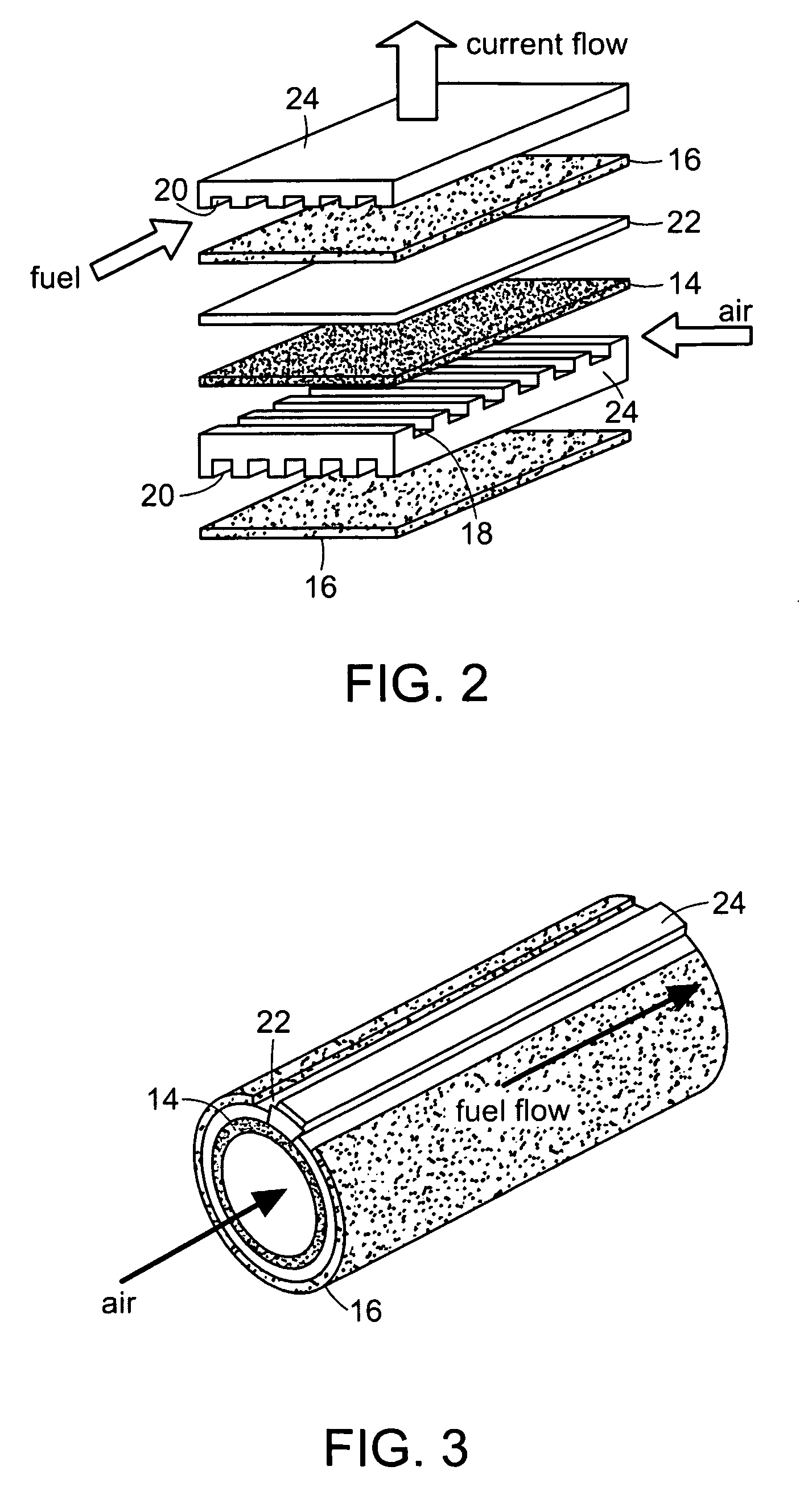
Ceramic interconnect for fuel cell stacks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Bi-layer Interconnect of La0.4Sr0.6Ti0.4Mn0.6O3−δ (“LSTM”) and Sr0.86Y0.08TiO3−δ (“YST”)
[0045]A small amount of (La,Mn)Sr-titanate, La0.4Sr0.6Ti0.4Mn0.6O3−δ (LSTM), powder (2.0 grams) was added on the top of (Nb,Y)Sr-titanate, Sr0.86Y0.08TiO3−δ (YST), powder (1.0 gram). The LSTM / YST powders were die-pressed together using a steel die with a diameter of 1.125 inches at a load of 10,000 lbs. The La0.4Sr0.6Ti0.4Mn0.6O3−δ powder was binderized before die-pressing with 0.5 wt % polyethylene glycol (PEG-400) and 0.7 wt % polyvinyl alcohol (PVA 21205) in order to increase the strength of the green body for handling. The die-pressed LSTM / YST powders with a bi-layer structure were then co-sintered pressurelessly at 1350° C. for one hour in air. The LSTM / YST bi-layer structure was cross sectioned, mounted in an epoxy, and polished for SEM (scanning electron microscope) examination. FIG. 4 shows an SEM result of the fabricated LSTM / YST bi-layer structure. As shown in FIG. 4, both LSTM and YST ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com