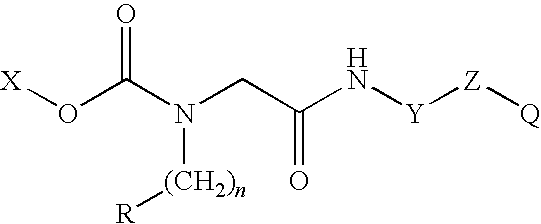
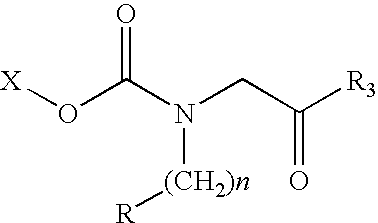
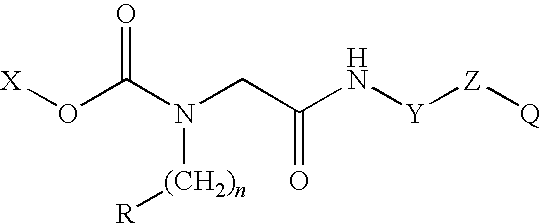
Conjugates of therapeutic or cytotoxic agents and biologically active peptides
a technology of biological active peptides and cytotoxic agents, which is applied in the field of conjugates of therapeutic or cytotoxic agents and biologically active peptides, can solve the problems of limiting the amount of active agent that can be administered to a patient, poor choice of commonly used ester derivatives, and inability to optimally linker strategies for peptide conjugates, etc., to achieve the effect of reducing the release and being easy to internaliz
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of the Chloroformate of Camptothecin
[0119]Camptothecin (250 mg) and 4-dimethylaminopyridine (DMAP; 50 mg) were suspended in 3 mL anhydrous pyridine and 50 mL anhydrous methylene chloride. Phosgene (750 μL of a 20% solution in toluene) was added to the slurry and mixed 2 h at ambient temperature. Excess phosgene and methylene chloride were evaporated in a chemical fume hood and the chlorformate of camptothecin dissolved in DCM.
example 2
Preparation of Camptothecin-carbonyl-N-aminoethyl-glycine-D-tert-butyl-Ser-Nle-D-tert-butyl-Tyr-D-tert-butyl-Ser-S- trityl-Cys-Phe-D-Trp -epsilon-tert-butyloxycarbonyl-Lys-tert-butyl-Thr-S-trityl-Cys-tert-butyl-Thr-Rink-amide-resin (SEQ ID NO: 16)
[0120]Rink amide [4-(2′,4′-dimethoxyphenyl-Fmoc-(aminomethyl)phenoxyacetamido-norleucyl-methylbenzhydrylamine resin (0.063 mmole), 100-200 mesh (Novabiochem, San Diego, Calif.) was added to the reaction vessel of a CS136 automatic peptide synthesizer (CS Bio, Inc., San Carlos, Calif.) and swollen in dimethylformamide (DMF) for approximately 1 hour. The resin was filtered and an excess of 20% piperidine in DMF was added and mixed (2 min). The resin was filtered and again an excess amount of 20% piperidine added and mixed (20 min) to ensure complete removal of the resin Fmoc group. After deprotection, the resin was washed 4 times with DMF and then the first protected amino acid, Fmoc-Thr(tBut) (0.188 mmol), diisopropylcarbodiimide (DIC) (0.1...
example 3
Preparation of camptothecin-carbonyl- N-(2-aminoethyl)-glycine-D-Ser-Nle-D-Tyr-D-Ser-cyclo[Cys-Phe-D-Trp-Lys-Thr-Cys]-Thr-amide (SEQ ID NO: 17)
[0122]The camptothecin-peptide resin prepared in EXAMPLE 2 (0.063 mmol) was placed in a round bottomed flask to which was added 15 mL of a solution of trifluoroacetic acid (TFA) containing water (2.5%), 1,2-ethanedithiol (2.5%), and triisopropylsilane (1%). The suspension was agitated (2 h) and filtered and washed several times with TFA. The TFA was evaporated in vacuo and ether added to the resulting oil to give a yellow powder that was then dissolved in 60% acetic acid (250 mL). A concentrated solution of iodine in methanol was added dropwise with vigorous stirring until a permanent brown coloration was formed whereupon excess iodine was removed by addition of a small quantity of ascorbic acid.
[0123]The solution was reduced to a volume of around 10 mL in vacuo and the crude camptothecin peptide purified by preparative reverse phase high pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com