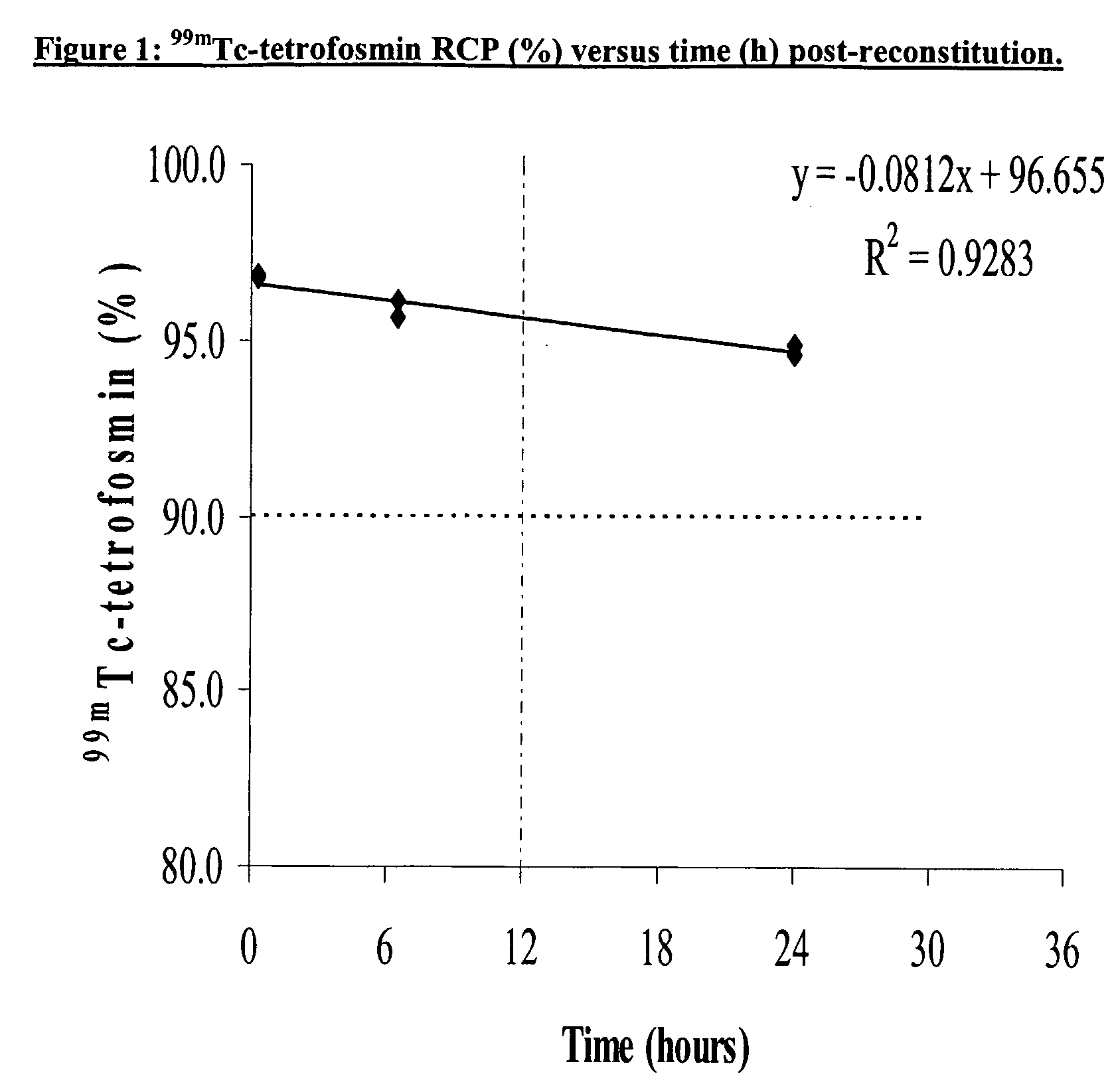
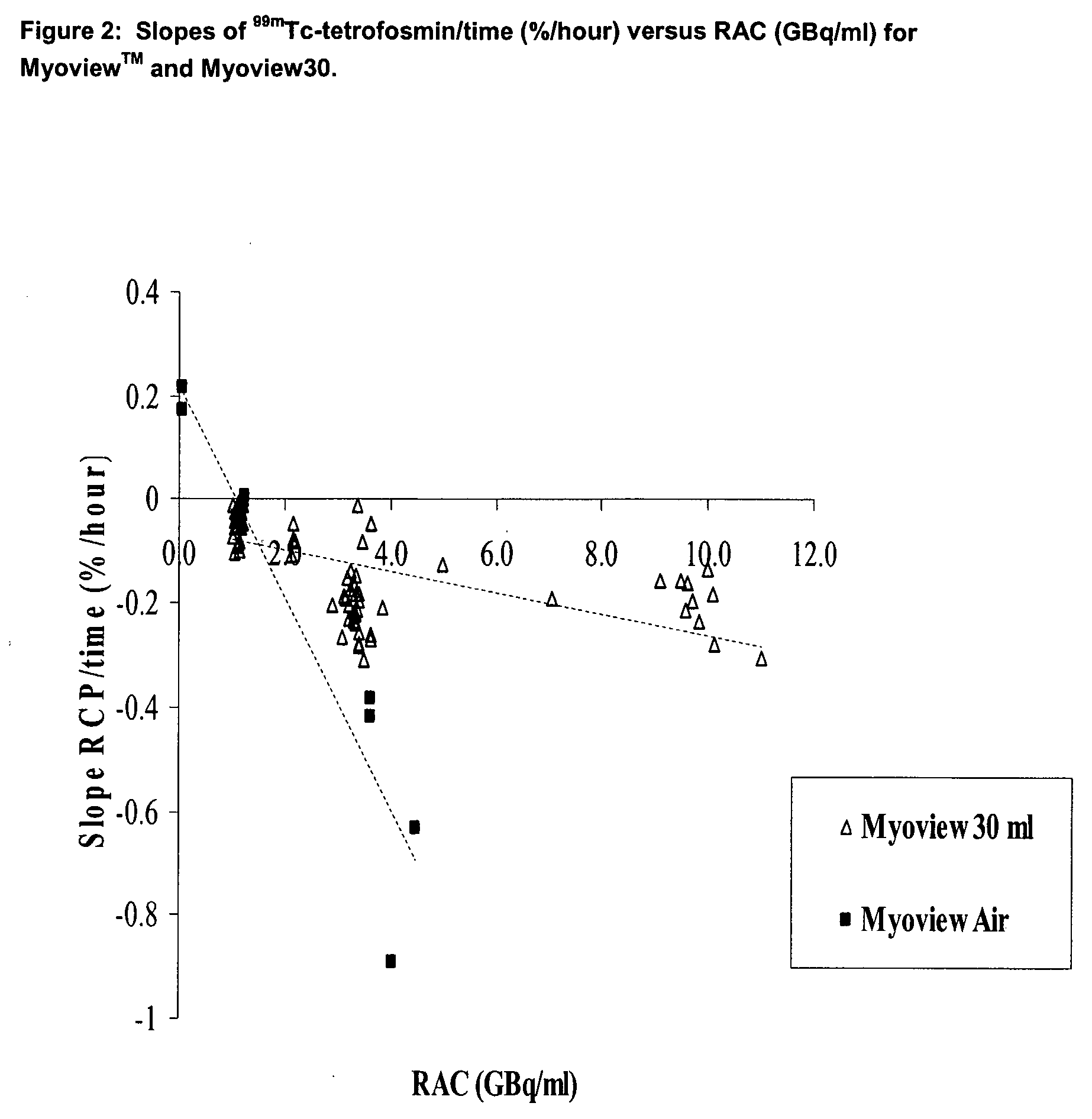
Stabalised 99mtc compositions
a radiopharmaceutical composition and composition technology, applied in the direction of radiation therapy, medical preparations, therapy, etc., can solve the problem of significant potential radiolysis problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Tetrofosmin
[0088]All reactions and manipulations were performed either in vacuo or under an oxygen-free nitrogen atmosphere. Solvents were dried, and degassed by nitrogen purge prior to use. α-Azo-isobutyronitrile (AIBN) and ethyl vinyl ether were obtained from BDH and Aldrich respectively. Bis(diphosphino)ethane was prepared according to the literature [Inorganic Synthesis, Vol 14, 10].
[0089]A Fischer pressure-bottle equipped with a Teflon™ stirring bar, was charged with ethyl vinyl ether (5 cm3, 52.3 mmol), bis(diphosphino)ethane (1 cm3, 10 mmol) and α-azo-isobutyronitrile (0.1 g, 0.61 mmol). The reaction mixture was then stirred and heated to 75° C. for 16 hours. After cooling back to room temperature, the viscous liquid was transferred to a 50 cm3 round-bottomed flask. Removal of volatile materials was performed by heating under vacuum. The involatile material obtained was pure by NMR. Yield: 3.0 g, 80%.
[0090]1H NMR (CDCl3): δ 1.12 (12H, dt J=1.16 Hz, 7.15 Hz; OCH2C...
example 2
Lyophilised Bulk Vial Kit Formulation and Preparation
[0092]An optimised formulation for a 30 ml bulk vial preparation is as follows:
Tetrofosmin0.69mg,Stannous chloride dihydrate90μg,Disodium sulfosalicylate0.96mg,Sodium-D-gluconate3.0mg,Ascorbic acid5.0mg,Sodium hydrogen carbonate11.0mg,pH on reconstitution with saline8.3 to 9.1.
[0093]This kit formulation is termed “Myoview30”.
[0094]Batches of 500 ml were prepared. Thus, approximately 90% of the total volume of Water for Injection (WFI) was added to a preparation vessel. The WFI was deoxygenated by purging with nitrogen. Tetrofosmin sulphosalicylate, stannous chloride dihydrate, Sodium D-gluconate, ascorbic acid and sodium hydrogen carbonate were dispensed, added and dissolved in consecutive order with constant mixing. The dispensing beakers were rinsed with deoxygenated WFI. The bulk solution was adjusted to 100% of the final volume with deoxygenated WFI during continuous mixing. The nitrogen purging was discontinued. A nitrogen bl...
example 3
Procedure for the Reconstitution of a Bulk Radioprotectant-Containing Kit
[0096]A Myoview30 kit 30 ml vial (from Example 2) was inserted into a suitable radioactive shielding container, and the rubber septum sanitised with an isopropyl alcohol swab. A sterile needle (the venting needle) was inserted through the rubber septum. 99mTc-pertechnetate generator eluate [10-30 cm3 volume; diluted with Sodium Chloride Injection, USP as appropriate which does not contain a bacteriostat; at a radioactive concentration of up to 10 GBq / cm3 and a total 99mTc radioactive content of up to 100 GBq (2.7 Ci)] was added using a shielded, sterile syringe. The venting needle was then removed. The reconstituted vial was mixed gently for 10 seconds to ensure complete dissolution of the lyophilised powder and then incubated at room temperature for 15 minutes.
[0097]The reconstituted Myoview30 was stored at 2-25° C., and the contents used within 12 hours of preparation. Withdrawn aliquots were also stored at 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com