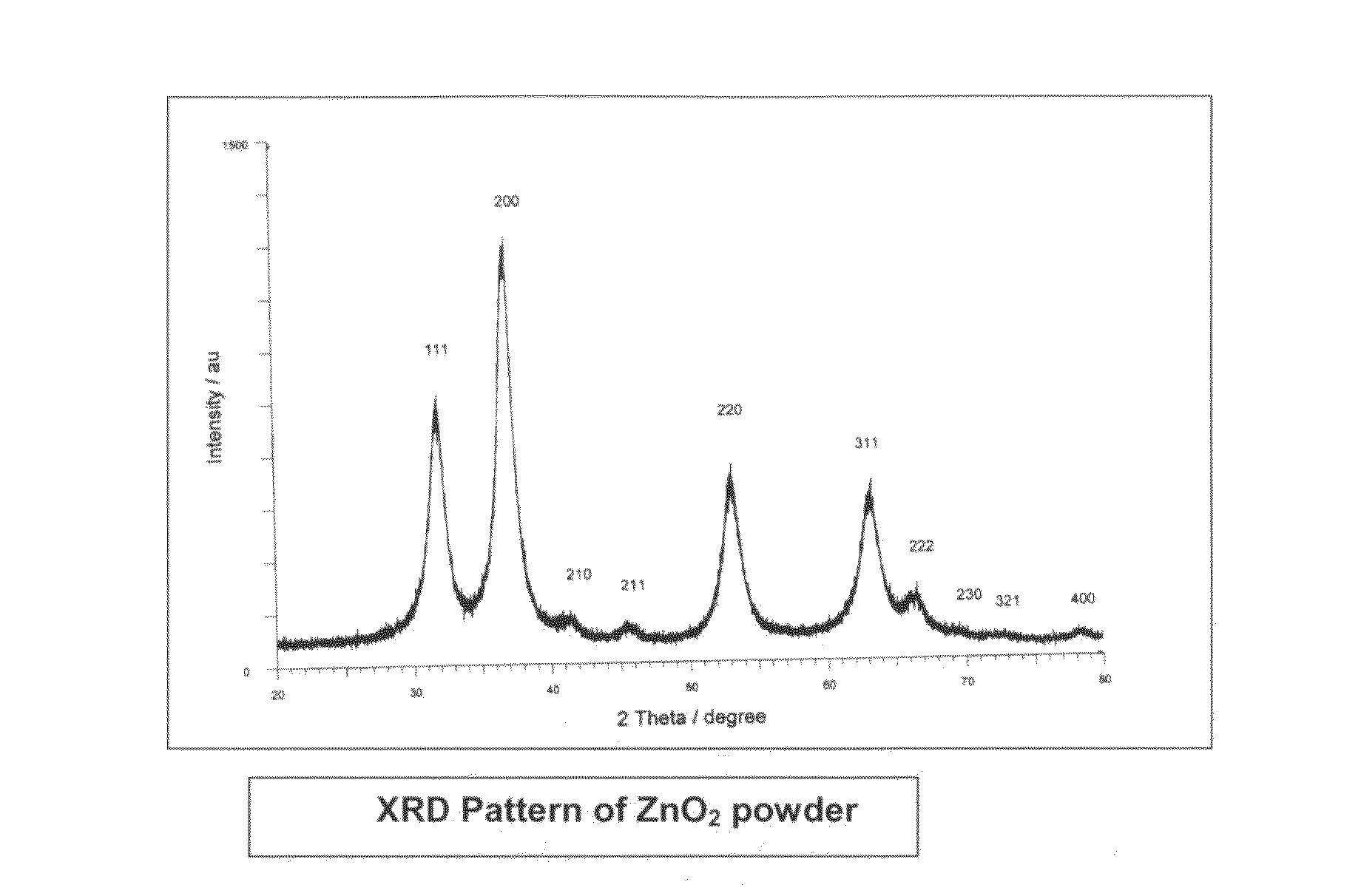
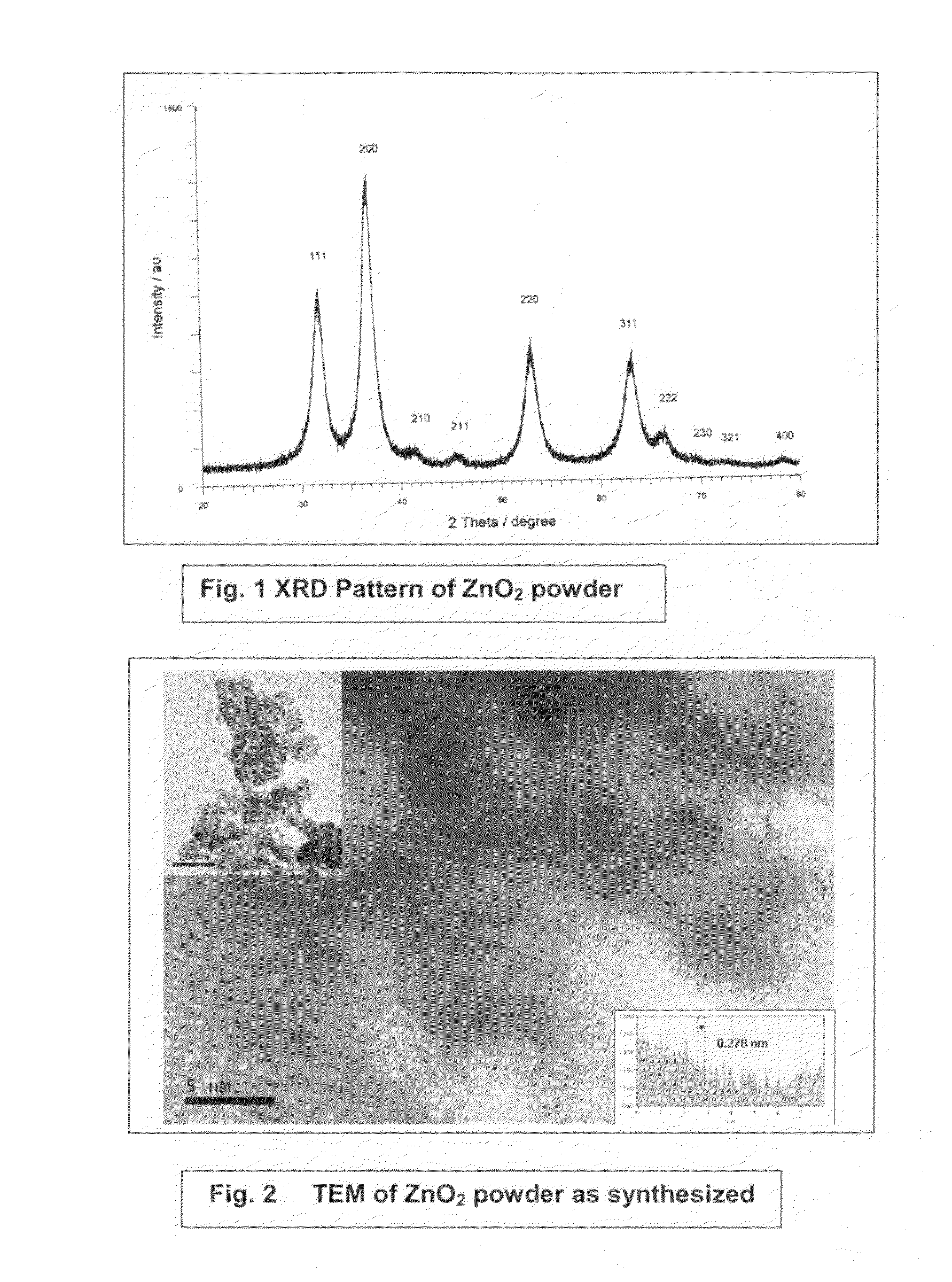
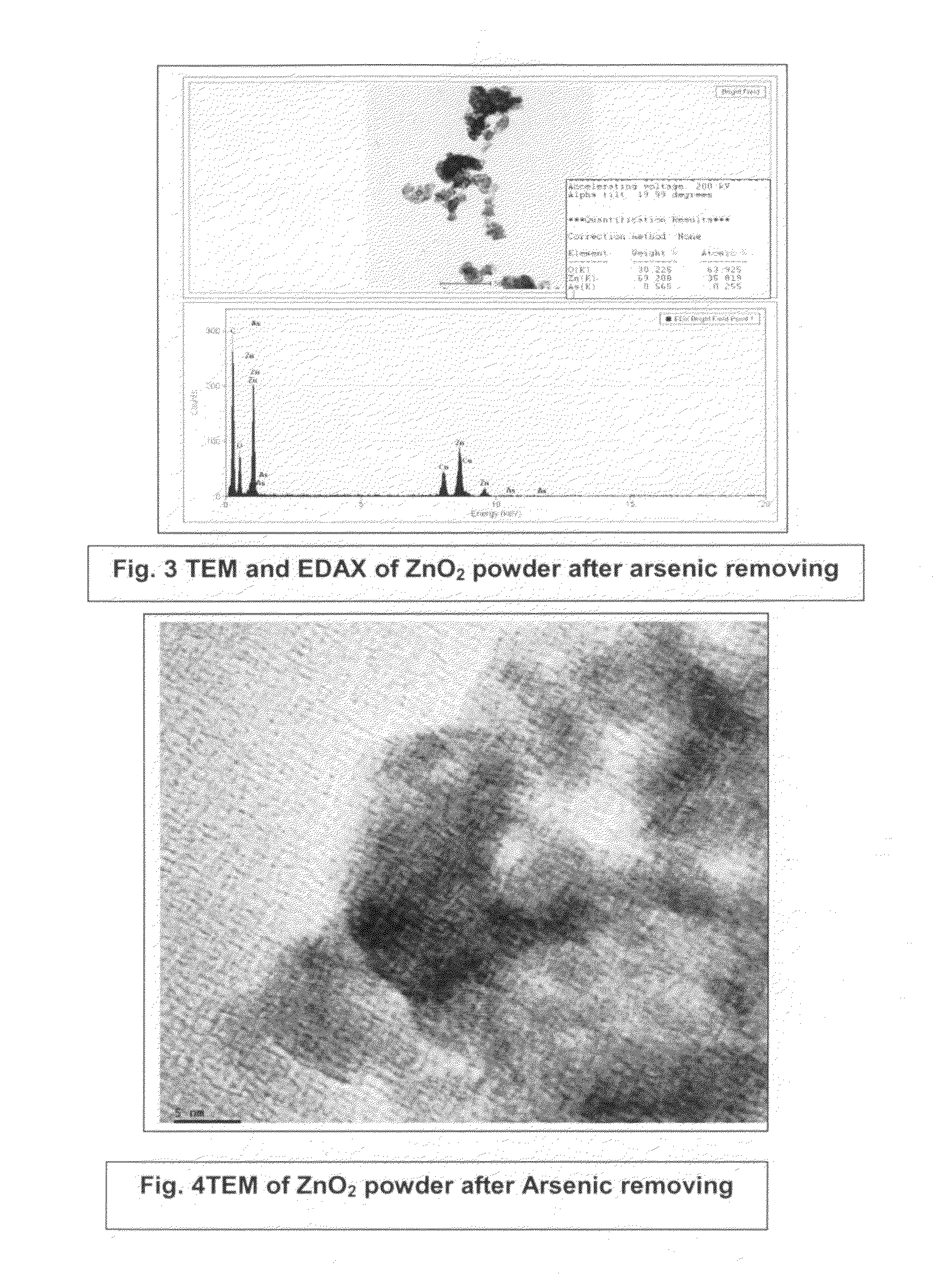
Process for the removal of arsenic and chromium from water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Methods for the preparation of ZnO2 nanoparticles using glycerol as surface modifier
[0072](i) 10 gm of zinc acetate was dissolved in 15 mL of ammonia solution and it was diluted to 200 ml of aqueous acetone (Water to solvent: 4:1). 2.5 gm of glycerol was added to the above solution mixture at pH 9.5 followed by adding 65 ml of hydrogen peroxide to obtain the nanoparticles of the zinc peroxide having average particle size distribution of 20±5 nm.
[0073](ii) 10 gm of zinc acetate was dissolved in 15 mL of ammonia solution and it was diluted to 200 ml of aqueous acetone (Water to solvent: 4:1). 5 gm of glycerol was added to the above solution mixture at pH 9.5 followed by adding 65 ml of hydrogen peroxide to obtain the nanoparticles of the zinc peroxide having average particle size distribution of 10±5 nm.
[0074](iii) 10 gm of zinc acetate was dissolved in 15 mL of ammonia solution and it was diluted to 200 ml of aqueous acetone (Water to solvent: 4:1). 0.5 gm of glycerol was added to th...
example 2
Preparation of ZnO2 nanoparticles using PVP as surface modifier
[0075](i) 10 gm of zinc acetate was dissolved in 15 mL of ammonia solution and it was diluted to 200 ml of aqueous methanol (Water to solvent: 4:1). 0.5 gm of PVP was added to the above solution mixture at pH 10, followed by adding 65 ml of hydrogen peroxide to obtain the nanoparticles of the zinc peroxide having average particle size distribution of 20±5 nm.
[0076](ii) 10 gm of zinc acetate was dissolved in 15 mL of ammonia solution and it was diluted to 200 ml of aqueous methanol (Water to solvent: 4:1). 1 gm of PVP was added to the above solution mixture at pH 10, followed by adding 65 ml of hydrogen peroxide to obtain the nanoparticles of the zinc peroxide having average particle size distribution of 10±5 nm.
[0077](iii) 10 gm of zinc acetate was dissolved in 15 mL of ammonia solution and it was diluted to 200 ml of aqueous methanol (Water to solvent: 4:1). 0.15 gm of PVP was added to the above solution mixture at pH 1...
example 3
Preparation of ZnO2 nanoparticles using TEA as surface modifier
[0078](i) 10 gm of zinc acetate was dissolved in 15 mL of ammonia solution and it was diluted to 200 ml of aqueous ethanol (Water to solvent: 4:1). 5gm of TEA was added to the above solution mixture at pH 11, followed by adding 65 ml of hydrogen peroxide to obtain the nanoparticles of the zinc peroxide having average particle size distribution of 20±5 nm.
[0079](ii) 10 gm of zinc acetate was dissolved in 15 mL of ammonia solution and it was diluted to 200 ml of aqueous ethanol (Water to solvent: 4:1). 10 gm of TEA was added to the above solution mixture at pH 11, followed by adding 65 ml of hydrogen peroxide to obtain the nanoparticles of the zinc peroxide having average particle size distribution of 10±5 nm.
[0080](iii) 10 gm of zinc acetate was dissolved in 15 mL of ammonia solution and it was diluted to 200 ml of aqueous ethanol (Water to solvent: 4:1). 1.5gm of TEA was added to the above solution mixture at pH 11, foll...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com