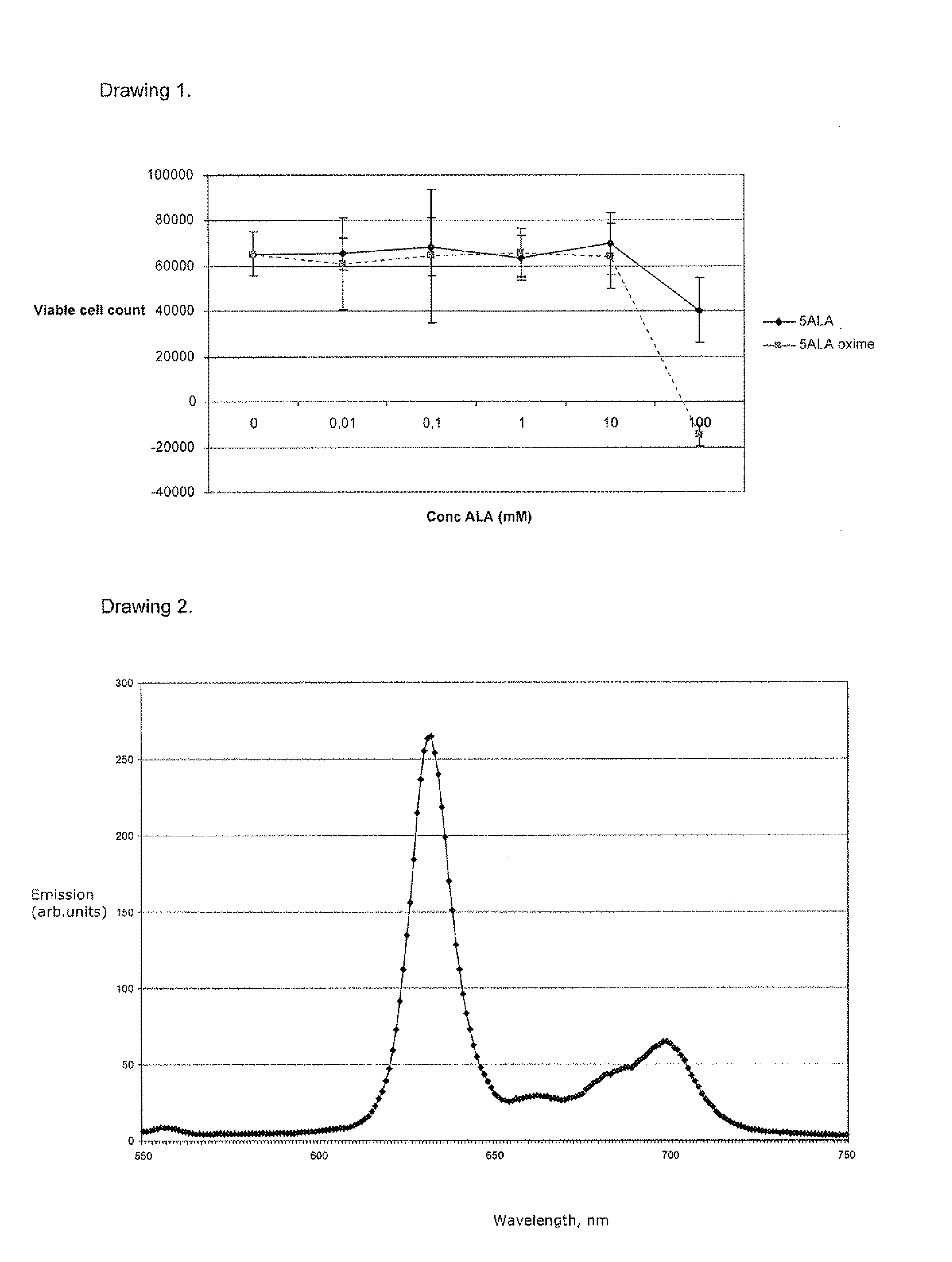
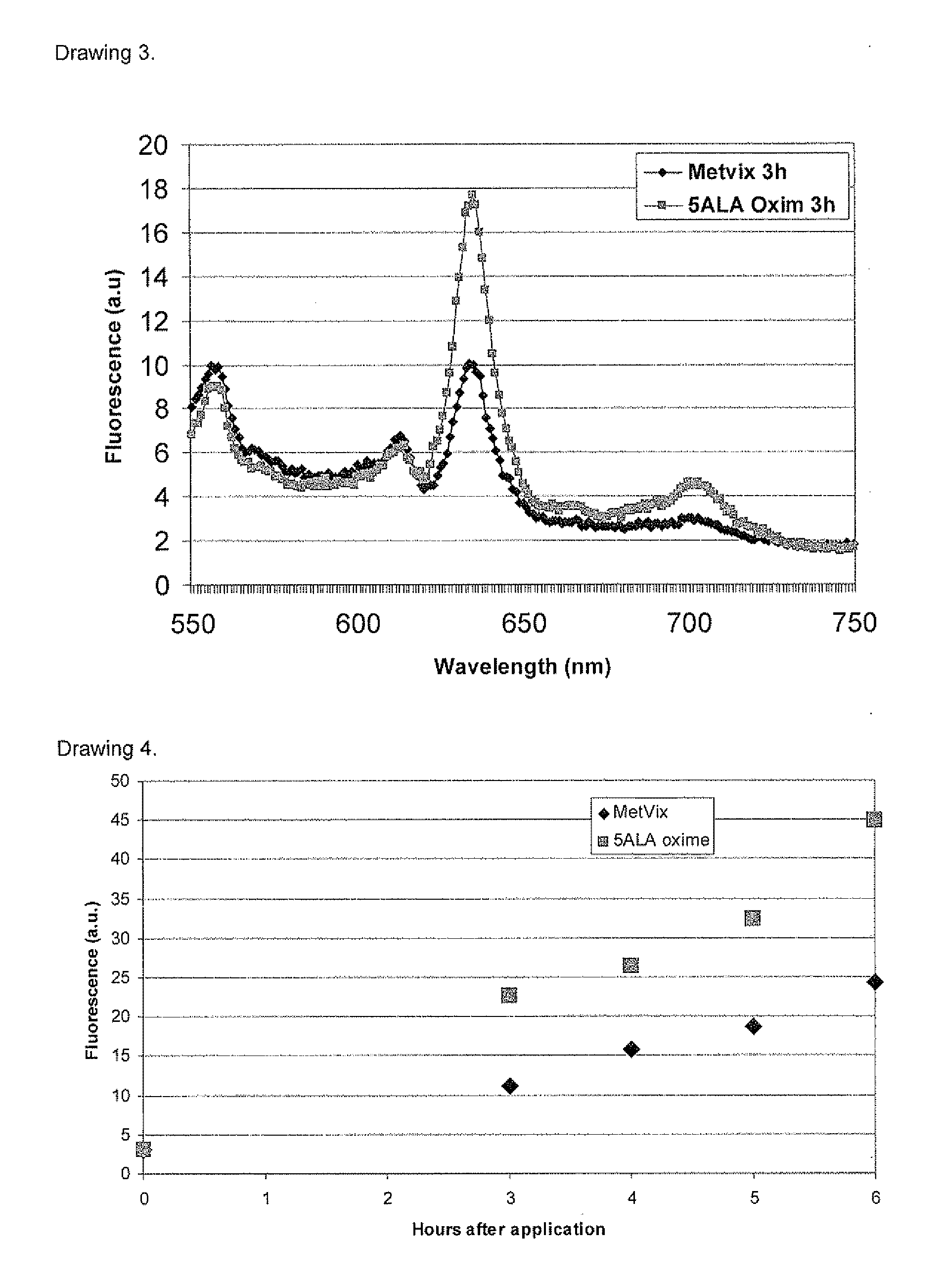
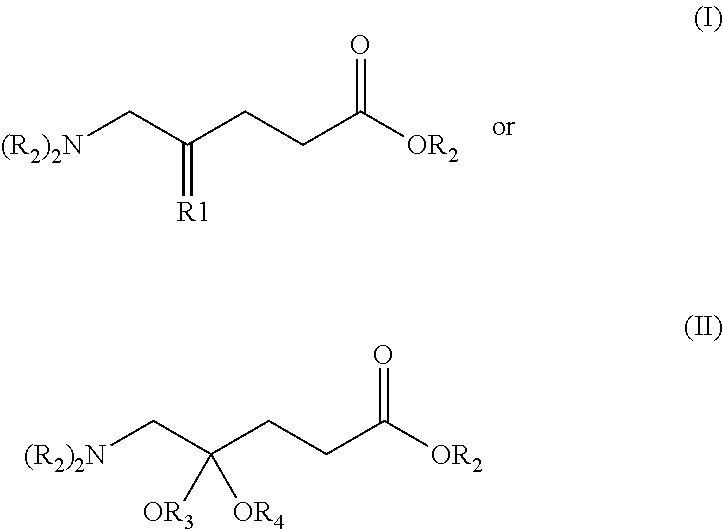
New 5-aminolevulinic acid prodrugs for use in photodynamic therapy and photodynamic diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0135]Synthesis of said compounds has been conducted according to two routes. The compounds used as starting materials herein are known from the literature, and in many cases commercially available, or may be obtained using methods known to the person skilled in the art. 5ALA, for example, is available from Sigma Aldrich.
A. Synthesis of 5-ALA oxime acid or corresponding methylated compound from 5-aminolevulinic acid or methyl-aminolevulinic acid
[0136]To a mixture of 5-ALA acid (or methyl-ALA) (3 g, 18 mmol) in EtOH (12 ml) at room temperature was added in one portion a clear solution of hydroxylamine hydrochloride (2.16 g, 31 mmol) and NaOAc (2.16 g, 26 mmol) in H2O (9.6 ml). The resulting mixture gave after a couple of minutes a clear solution and was stirred at reflux for 1.5 hours after which time the reaction was completed according to 1H NMR.
[0137]After the clear slightly yellow solution had cooled to room temperature, EtOH was removed from the reaction under reduced pressure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com