Hyperlipemia-ameliorating agent, anemia-ameliorating composition, uric-acid-level-reducing composition, and food or beverage
a technology of anemia and ameliorating agent, which is applied in the direction of drug compositions, extracellular fluid disorders, metabolic disorders, etc., can solve the problems of increased creatine kinase (cpk) level, deterioration of high blood pressure condition, and difficulty in selecting optimal diet restriction and exercise therapy methods, etc., to achieve excellent neutral fat level regulation, excellent anemia-ameliorating effect, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example 1
Production of Hyperlipemia-Ameliorating Agent
[0164]5.9% by mass hydrochloric acid (6,666 mL) and 99.9% by mass aqueous ethanol solution (3,334 mL) were added to Panax notoginseng powder (1,000 g, product of MATSUURA YAKUGYO CO., LTD.) containing saponins such as ginsenoside-Rg1, notoginsenoside-R1, ginsenoside-Re, ginsenoside-Rb1, ginsenoside-Rd and ginsenoside-Rc. The resultant suspension was hydrolyzed with heating for 6 hours at 80° C. to prepare an aglycon-containing extract. Then, the pH of the aglycon-containing extract was adjusted to 6.7 with 6.6M aqueous sodium hydroxide solution to reduce the ethanol concentration, followed by suction filtration.
[0165]The residue was dried with heating under reduced pressure, to thereby obtain a strong acid-treated Panax notoginseng product containing aglycons.
example 1
Study on Hyperlipemia-Ameliorating Effects
—Preparation of Feed—
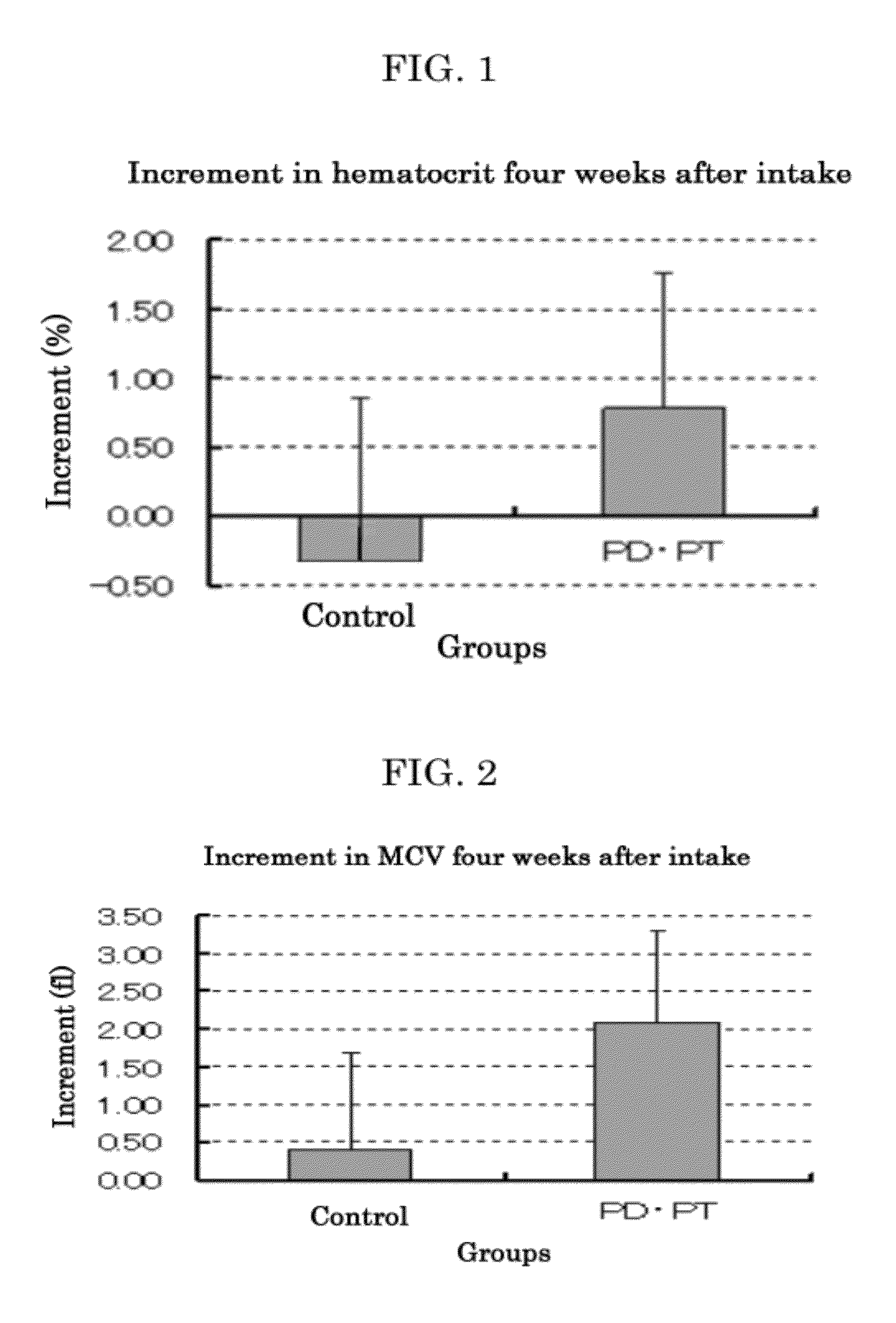
[0166]Protopanaxatriol (PPT) (product of Funakoshi Corporation), panaxatriol (PT) (product of Funakoshi Corporation), protopanaxadiol (PPD) (product of Funakoshi Corporation) and panaxadiol (PD) (product of Funakoshi Corporation) were each added to feed CE2 (product of CLEA Japan, Inc.) so that the concentration thereof was 0.05% by mass.
[0167]Similarly, the strong acid-treated Panax notoginseng product (Production Example 1) and Panax notoginseng powder (product of MATSUURA YAKUGYO CO., LTD.) were each added to feed CE2 (product of CLEA Japan, Inc.) so that the concentration thereof was 1.0% by mass.
[0168]Feed CE2 (product of CLEA Japan, Inc.) containing no other additives was used as feed for the control group.
—Intake—
[0169]Type 2-diabetes model mice KK-Ay / Ta Jc1 (male, 4 weeks old, 8 mice / group, product of CLEA Japan, Inc.) were preliminarily bred for 7 days. Thereafter, they were bred for 5 days with the above-prepar...
example 2-1
[0182]A capsule was charged with 1.0 g of crystalline cellulose containing 0.6% by mass of panaxadiol (product of LKT Laboratories, Inc.) and 1.4% by mass of panaxatriol (product of LKT Laboratories, Inc.) (the total amount of panaxadiol and panaxatriol: 20.0 mg) to prepare an anemia-ameliorating composition of Example 2-1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com