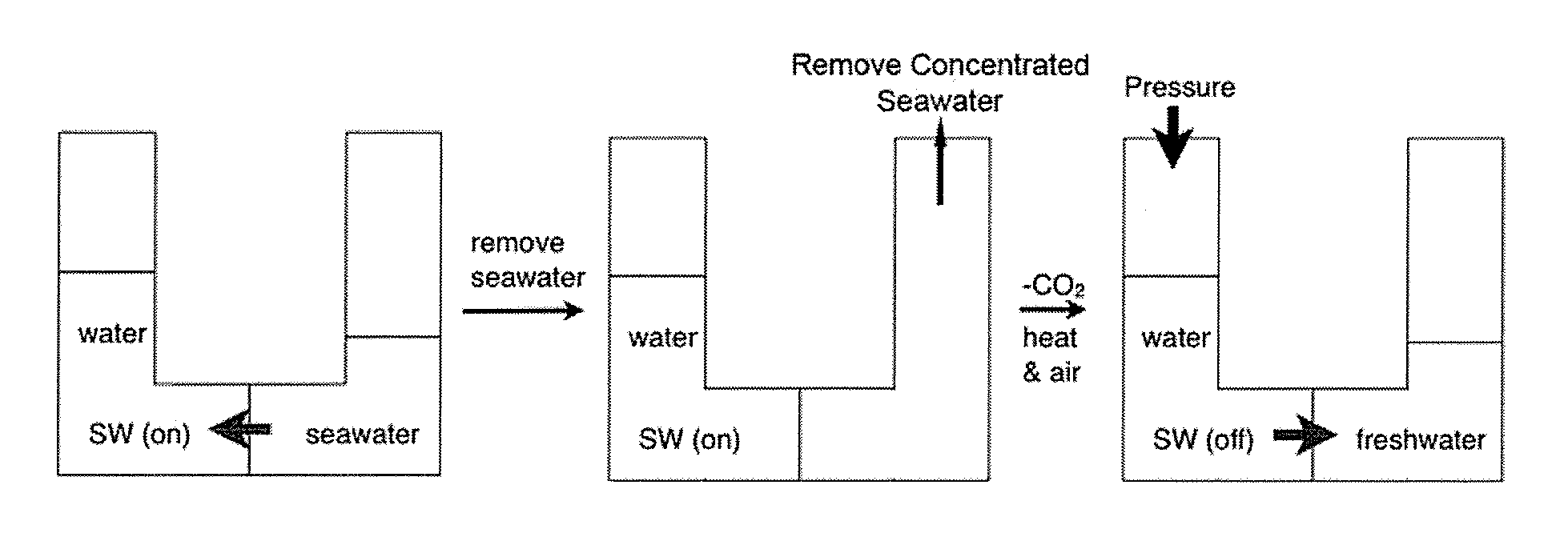
Water with Switchable Ionic Strength
a technology of ionic strength and water, applied in the field of solvent composition, can solve the problems of significant limitations in the use of ionic strength as a media for reaction and separation, adds to the economic cost and environmental impact of the overall process, and is difficult to achieve, so as to reduce the cost of ionic strength. , the effect of modulating ionic strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
working examples
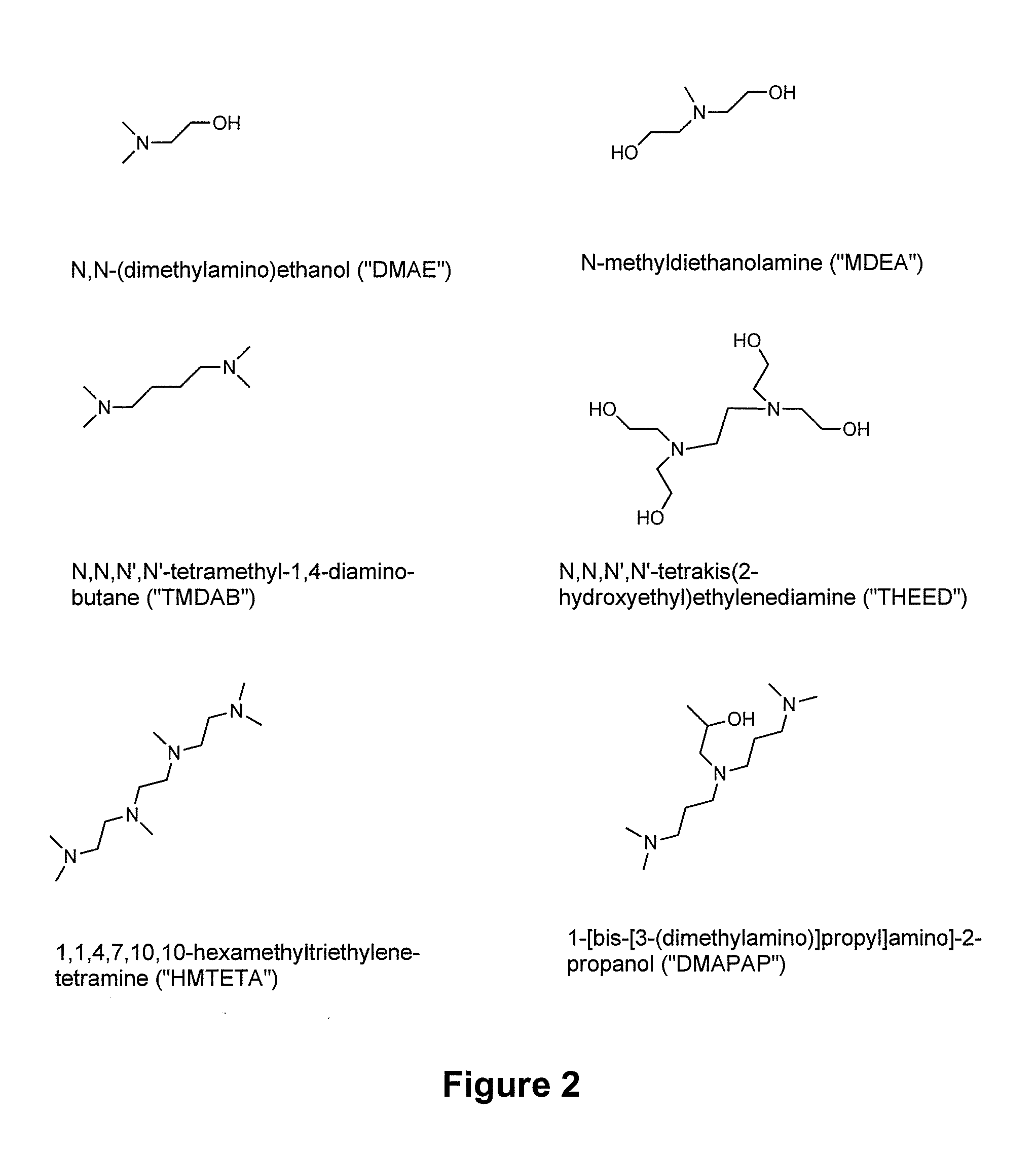
[0301]The following chemicals were used as received: ethanolamine, 2-(methylamino) ethanol, chloroform-d (99.8+atom % d), D2O (99.9+atom % d), acetonitrile-d3 (99.8+atom % d), methanol-d4 (99.8+atom % d), 1,4-dioxane (99+%), DMAE, MDEA, TMDAB, THEED, DMAPAP and HMTETA (Sigma-Aldrich of Oakville, Ontario, Canada, “Aldrich” or TCI of Portland, Oreg., USA); THF (99+%) and ethyl acetate (99.5+%) (Caledon Laboratories, Ontario, Canada); hydrochloric acid (˜12 M, Fischer Scientific, Ottawa, Ontario, Canada); and DMSO-d6 (99.9+atom % d) Cambridge Isotope Labs, St Leonard, Canada).
[0302]Diethyl ether was purified using a double-column solvent purification system (Innovative Technologies Incorporated, Newbury Port, USA). Compressed gasses were from Praxair (Mississauga, Ontario, Canada): 4.0 grade CO2 (99.99%), 5.0 grade Ar (99.999%), supercritical grade CO2 (99.999%, H2O2O2O<5 ppm).
[0303]Unless otherwise specified, water used in studies described herein was municipal tap water from Kingston...
example 1
Reversible Solvent Switching in Tertiary Amine / Water Systems
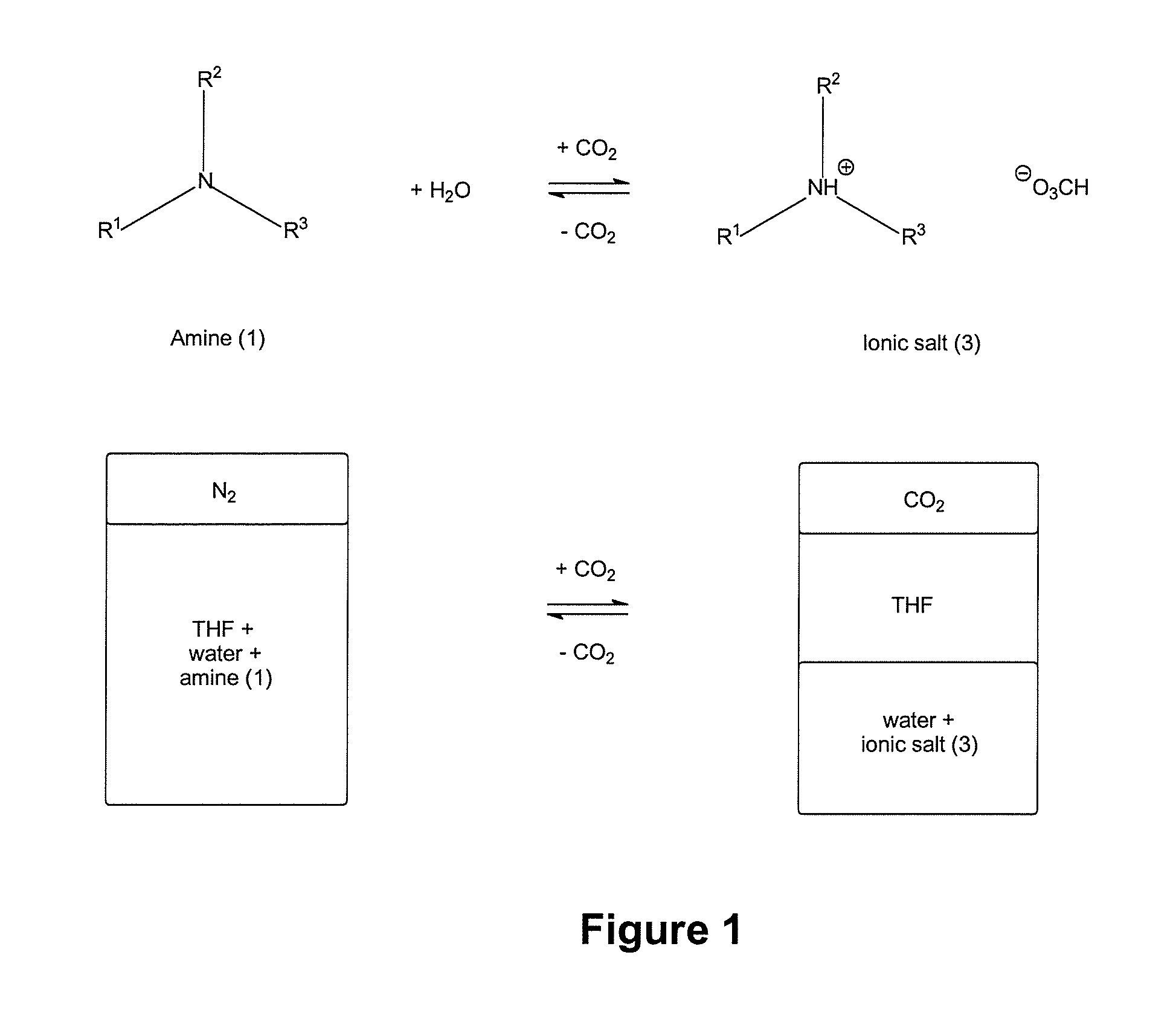
[0318]Three tertiary amines, DMAE, MDEA and THEED were investigated as additives for switchable ionic strength solutions. DMAE and MDEA are monoamines, and THEED is a diamine.
[0319]Six dram vials containing a magnetic stirrer and fitted with a rubber septa were prepared with 1:1:1 w / w / w solutions of water, THF, and an additive of tertiary amine compound of formula (1). To introduce gas to the solution, a single narrow gauge steel needle was inserted and gas was bubbled through. A second narrow gauge steel needle was inserted to allow venting of the gaseous phase.
[0320]The solutions were tested for switchable ionic strength character by bubbling CO2 through the mixtures. The time necessary to observe separation of the THF from the aqueous solution of the ionic bicarbonate salt was recorded and is shown in Table 1. It was determined that it typically takes 30 min of bubbling with CO2 to separate out THF from the aqueous phase...
example 2
Quantitative Determination of the Separation of Compound and Additive Upon Switching
[0323]The three switchable aqueous solution systems of Example 1 were further investigated by 1H NMR spectroscopy to quantify the amount of THF separated from the aqueous phase upon switching of the additive to its higher ionic strength ammonium bicarbonate form, and to quantify the amount of additive retained in the aqueous solution after switching.
[0324]To measure the extent of THF being forced out of an aqueous phase by an increase in ionic strength, and the amounts of amine which remained in the aqueous phase, 1:1:1 w / w / w solutions of water, THF, and amine additive were prepared in graduated cylinders and the cylinders were capped with rubber septa. After 30 minutes of bubbling CO2 through the liquid phase at a flow rate of 3-5 ml min−1) as measured by a J&W Scientific ADM 2000 Intelligent Flow Meter, from a single narrow gauge steel needle, a visible phase separation was observed. The volumes of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pKa | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com