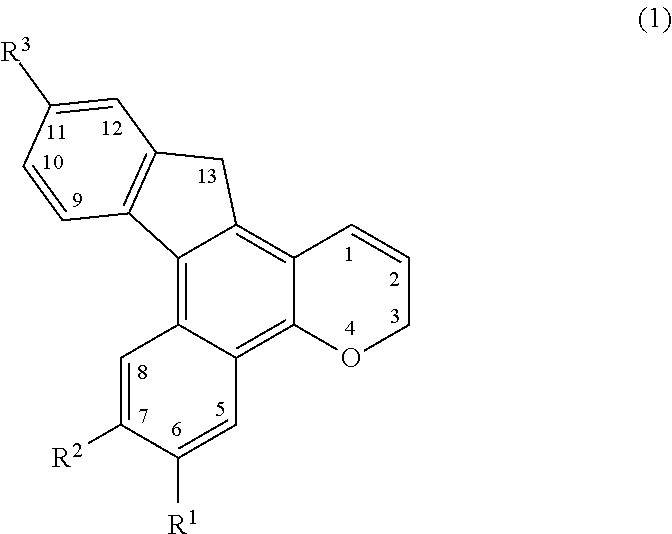
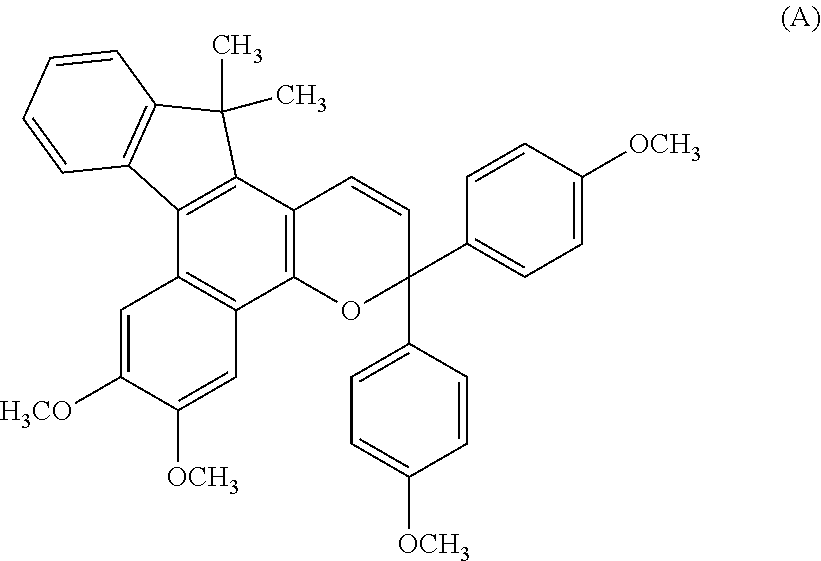
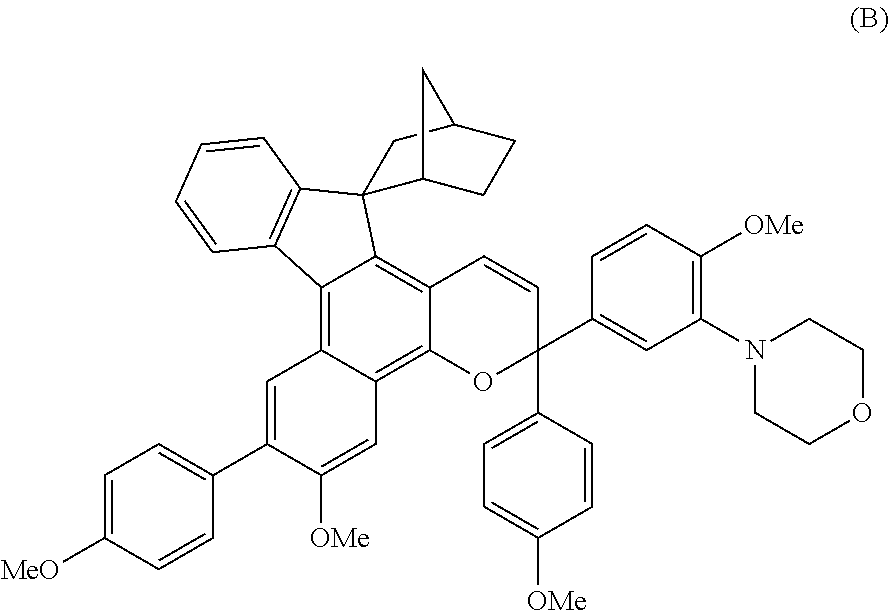
Chromene compound
a chrome compound and compound technology, applied in the field of chrome compound, can solve the problems of low durability, low fading speed, and strong color development, and achieve the effects of low initial coloration, high double peak characteristic, and high color development sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0202]1.20 g (2.0 mmol) of a naphthol compound represented by the following formula (22) and 0.80 g (3.0 mmol) of a propargyl alcohol compound represented by the following formula (23) were dissolved in 70 ml of toluene, 0.022 g of p-toluenesulfonic acid was further added to the resulting solution, and the obtained mixture was stirred under reflux by heating for 1 hour.
After a reaction, the solvent was removed, and the obtained product was purified on silica gel by chromatography to obtain 1.35 g of a white powder product. The yield was 80%.
[0203]The elemental analysis values of this product were 78.22% of C, 6.72% of H, 1.55% of N and 3.75% of S which were almost equal to the calculated values of C52H52O6 (C, 78.26%, H, 6.81%, N, 1.66%, O, 9.48%, S: 3.80%)
[0204]When the proton nuclear magnetic resonance spectrum of the product was measured, it showed 18H peaks based on the methyl proton and methylene proton of a tetramethylcyclohexane ring at δ of around 1.0 to 3.0 ppm, 20H peaks b...
examples 2 to 16
[0206]Chromene compounds shown in Tables 1 to 5 were synthesized in the same manner as in Example 1. When the structures of the obtained products were analyzed by using the same structure confirming means as in Example 1, it was confirmed that they were compounds represented by structural formulas shown in Tables 1 to 5. Table 6 shows the elemental analysis values, calculated values obtained from the structural formulas and characteristic 1H-NMR spectra of these compounds.
TABLE 1Raw materialsEx-PropargylampleNaphtholalcoholYieldNo.compoundcompoundProduct(%)277375470
TABLE 2Raw materialsEx-PropargylampleNaphtholalcoholYieldNo.compoundcompoundProduct(%)573674768
TABLE 3Raw materialsEx-PropargylampleNaphtholalcoholYieldNo.compoundcompoundProduct(%)8719681066
TABLE 4Raw materialsEx-PropargylampleNaphtholalcoholYieldNo.compoundcompoundProduct(%)116112721366
TABLE 5Raw materialsEx-PropargylampleNaphtholalcoholYieldNo.compoundcompoundProduct(%)146815601664
TABLE 6Elemental analysis valuesExampl...
example 17
[0207]1.0 g (2.1 mmol) of the following naphthol compound (25) and 0.80 g (3.0 mmol) of the above propargyl alcohol compound (23) were dissolved in 70 ml of toluene, 0.022 g of p-toluenesulfonic acid was further added to the resulting solution, and the obtained mixture was stirred under reflux by heating for 1 hour.
After a reaction, the solvent was removed, and the obtained product was purified on silica gel by chromatography to obtain 1.1 g of a white powder product. The yield was 72%.
[0208]The elemental analysis values of this product were 75.68% of C, 6.70% of H and 8.93% of S which were almost equal to the calculated values of C45H46O4S (C, 75.79%, H, 6.64%, S: 8.80%)
[0209]When the proton nuclear magnetic resonance spectrum of the product was measured, it showed 18H peaks based on the methyl proton and methylene proton of a tetramethylcyclohexane ring at δ of around 1.0 to 3.0 ppm, 15H peaks based on the methyl proton of a methylthio group and the methyl proton of a methoxy grou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
| optical density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com